S0002020
Sterilizer,steam,20L,electric,w/access
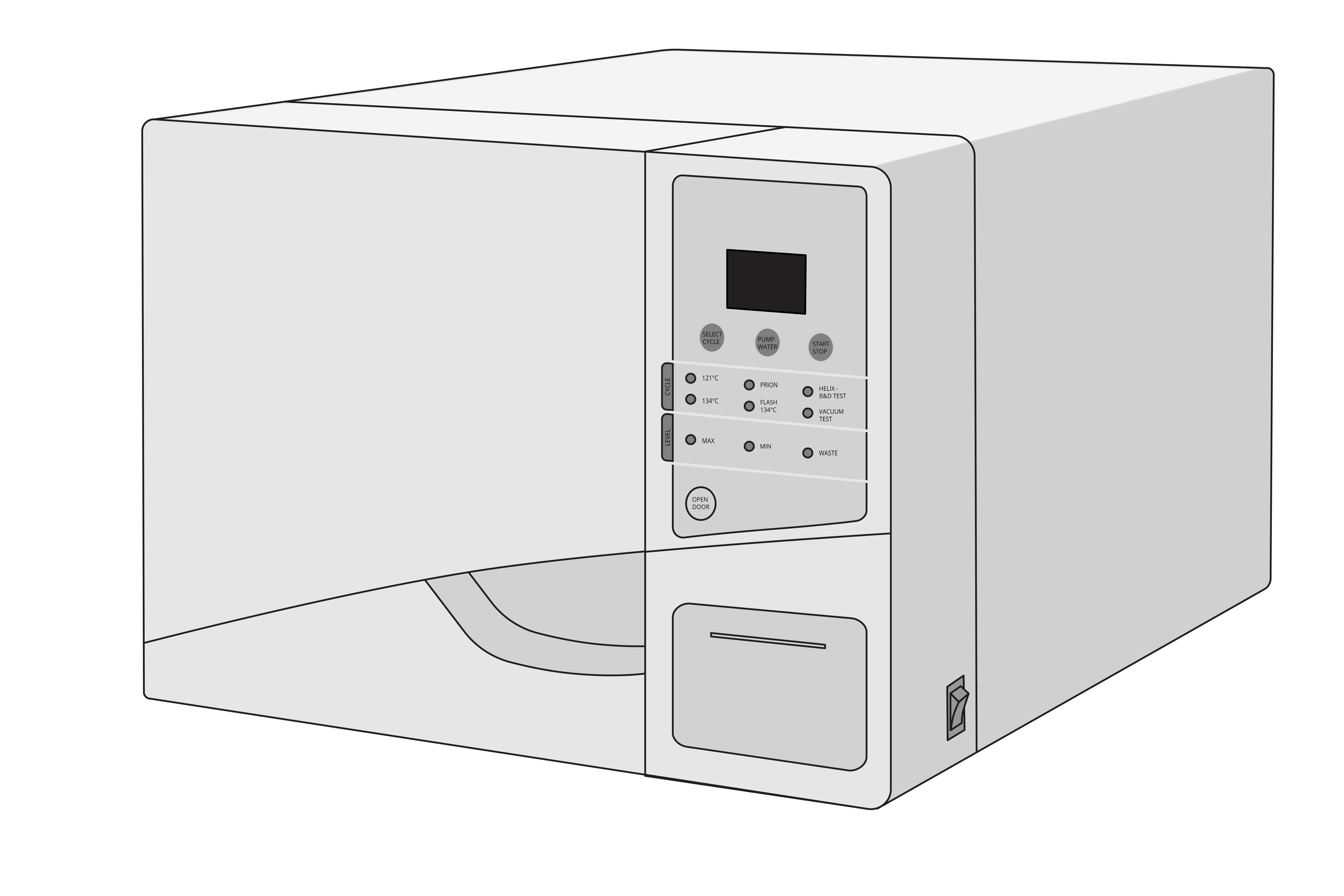
Electric tabletop steam sterilizer (autoclave) with a minimum capacity of 20 Litres including accessories. This unit is suitable for sterilization of full solid, porous, hollow A and B, plastics, rubber, unwrapped, bagged, single or double wrapped. Not designed for sterilization of liquids, food stuffs or waste.
Indicative Price 5,750.00 USD
GENERAL DESCRIPTION
Tabletop electric sterilizer (autoclave) with a capacity between 20 and 22 litre
IMPORTANT: To ensure the sterilizer is capable to perform as per manufacturers specifications certain infrastructure provision are required. Therefore, these sterilizers should only be purchased after consultation of the Medical Devices Unit in Supply Division.
INTENDED USE
Steam sterilizers use pressurized steam to generate moist heat to eliminate viable microbes from non-heat-sensitive medical devices, including heat-tolerant products used for surgical and general patient care. Steam sterilization kills microbes effectively and is often viewed as the gold standard of healthcare sterilization methods. For sterilization of full solid, porous, hollow A and B, plastics, rubber, unwrapped, bagged, single or double wrapped. Not designed for sterilization of liquids, food stuffs or waste.
TECHNICAL SPECIFICATIONS
The internal chamber is made from stainless steel 316L.
The internal chamber has a volume between 20 and 22L.
Vacuum relief line should be filtered with ≤0.3 µm pore size to allow sterile air into chamber.
The unit has at least one 3 internal shelfs/trays.
The unit has a standard program at 2.2 bar at a temperature of 134°C.
The unit has a standard program between 1.0 and 1.1 bar with a temperature between 118 – 121°C.
The unit is fitted with a safety lock on the front door, preventing the door to be opened when the unit is under pressure.
The unit is provided with an automatic cycle shut-off.
The unit provided with an internal water tank with a minimum capacity of 4 litres.
The unit equipped with a steam generator.
The unit is fitted with a water circuit filter.
The unit is designed in such a way that outer surfaces remain at safe temperatures when the unit is in operation.
The unit is designed and constructed in such a manner that it is able to withstand frequent disinfection with hospital-grade products.
The unit's power requirements do not exceed 3 kW.
The unit work on a single-phased power grid.
Power requirements: 230 Volts - 50/60 Hz. (110 Volt units are not available).
DISPLAY FEATURES
The unit is fitted with a front panel providing information on the unit.
The display indicates:
- The time until the programme is finished.
- The current internal temperature.
- The current internal pressure.
- The ongoing cycle
- Any alerts or alarms.
ALARM AND SAFETY FEATURES
Alarms are audible as well as visual.
Alarms are available for/when:
- Low water.
- Door not closed.
- Exceeding high temperature limit.
- Failed cycles.
- Temperatures are about to reach limits of the equipment.
- Pressures are about to reaching limits of the equipment.
- Power failure
A notification that the end of sterilization cycle has been reached.
The unit is provided with an over-pressure safety valve and an over-temperature safety system.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, other languages on request.
1 x Plastic protective dustcover.
3 x sterilizer baskets.
3 x spare gaskets (chamber/door) in sealed packages.
1 x set of spare fuses, if required.
OPTIONAL ACCESSORIES AVAILABLE
At extra costs additional optional features can be requested:
- Filtration system or distiller solution which will allow the unit to accept so-called "hard water", (water with high mineral content, especially calcium and magnesium) without causing internal damage and/or deposits.
ESTIMATED LIFE SPAN
10 years
WARRANTY
Two years from shipping date
ENVIRONMENTAL CONDITIONS
- Operating conditions: 5°C - 40°C / 50% – 80% RH
- Storage conditions: 0°C - 50°C / 90% RH
Depending on the model:
- Suitable for operations up to an altitude above sea level of 2,000m
or
- Suitable for operations in atmospheric pressure: 750 ~ 1100 hPa
WEIGHT AND VOLUME
Weight: 62 kg (packaged)
Volume: 270.00 dm³ (packaged)
ESTIMATED DELIVERY LEAD TIME
100 days
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
ITEMS REQUIRED, BUT NOT SUPPLIED
S0167100 Papersheet,crepe,for ster. pack/PAC-100
S0558100 Indicator,TST control spot/PAC-300
S0167110 Masking tape,for ster. pack
ALTERNATIVE PRODUCTS
S0002022, Sterilizer,steam,40L,electric,w/access
S0004045, Sterilizer,steam,large,electric,waccess
S0156000, Sterilizer,steam,39L
S0157000, Sterilizer,steam,24L
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 61010-2-040:2015 Safety requirements for electrical equipment for measurement, control, and laboratory use - Part 2-040: Particular requirements for sterilizers and washer-disinfectors used to treat medical materials.
- ISO 14971 Medical devices -- Application of risk management to medical devices.
- ISO 17665-1 (replacing ISO 13683 and ISO 11134) Sterilization of health care products -- Moist heat -- Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices.
- EN 13060:2014+A1:2018 Small Steam Sterilizers (Less than 60 L).
NOMENCLATURE
- GMDN code: Steam sterilizer (38671)
- UMDNS code: Sterilizing Units, Steam, Tabletop (16142), Sterilizing Units, Steam (13746)
Sterilizing Units (13737)
Related Products