S0002069
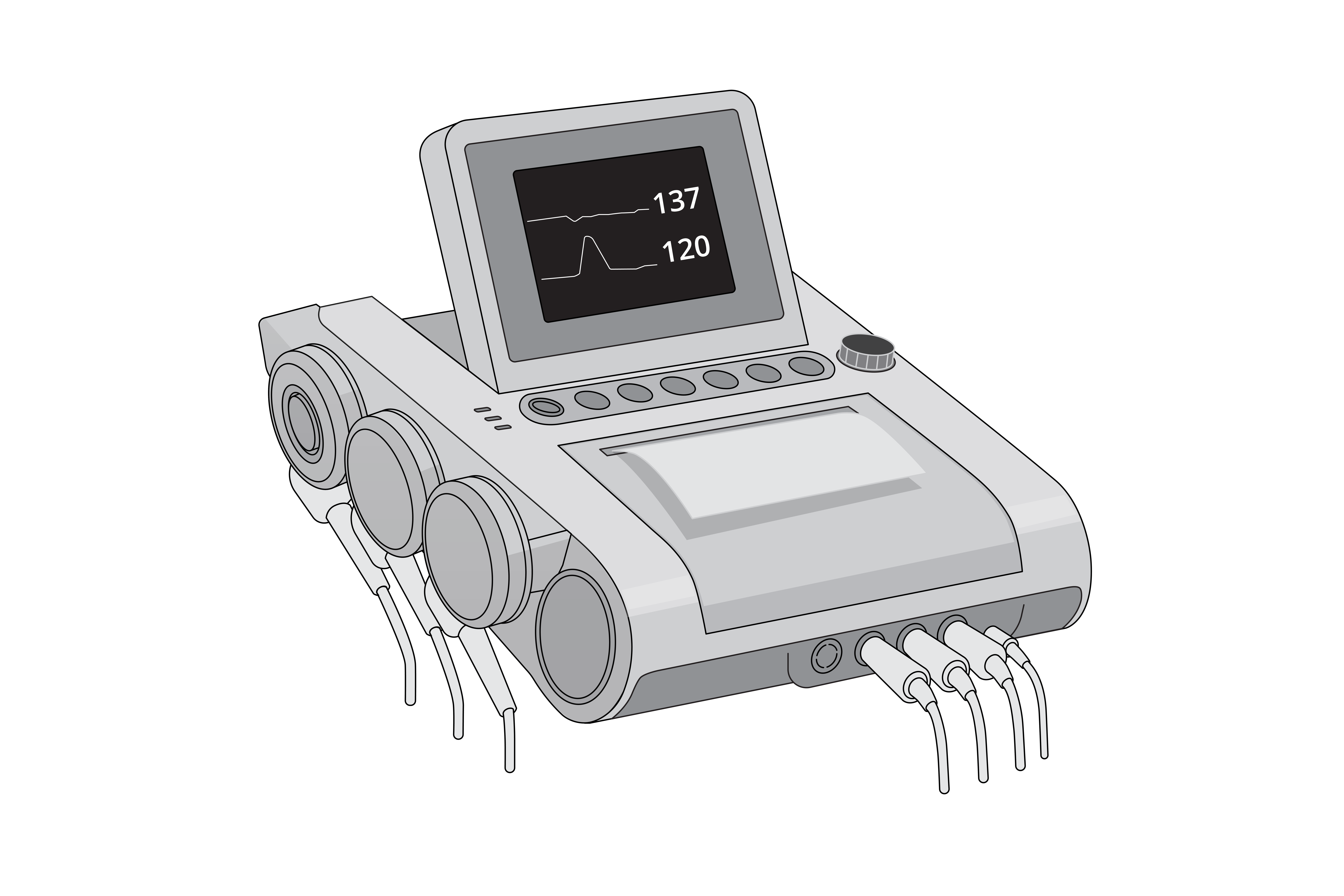
CTG monitor,with accessories
Cardiotocograph (CTG monitor), also refereed to as an antepartum foetal monitor). This tabletop unit includes normal and twins Foetal Heart Rate (FHR) and Uterine Contraction (UC) detection, is AC and battery powered and fitted with a loudspeaker, an event marker, a printer and supplied including accessories.
Indicative Price 1,364.00 USD
GENERAL DESCRIPTION
Cardiotocograph (CTG) system for antepartum foetal and maternal monitoring.
INTENDED USE
Electronic foetal monitoring (EFM) provides graphic and numeric information on foetal heart rate (FHR) and maternal uterine activity (UA) to help clinical personnel assess foetal well-being. During labour, the FHR often exhibits decelerations and accelerations in response to uterine contractions or foetal movements; certain patterns are indicative of hypoxia. Examination of these patterns, the baseline level, and variability characteristics can indicate the need to alter the course of labour with drugs or perform an operative delivery (caesarean section or forceps delivery) if corroborated by other clinical evidence. Foetal monitors can also provide documentation of the foetus’s condition that could be useful in the event of litigation.
Antepartum monitors are used to monitor the foetus’s development, movement, and FHR patterns in utero. Antepartum monitors have only external monitoring capabilities, such as ultrasound and external UA, and are typically used in the physician's office or clinic. They also are often used in the hospital for high-risk mothers who are hospitalized before term for observation or treatment not in conjunction with labour.
TECHNICAL SPECIFICATIONS
Capable of external monitoring of foetal heart rate (FHR) and maternal uterine activity (UA).
Alphanumeric display shows FHR1, FHR2 and UCs and alarms.
Automatically detects transducers when they are plugged in.
Includes remote switch for event marking.
Automatic self-test on power up.
System reports with status and alarms.
Unit includes a battery allowing for > 2 hours continuous operation.
Power requirements: 100 - 240 Volts - 50/60 Hz.
FETAL HEART RATE MONITORING
Foetal heart rate detected by ultrasonic transducer/probe.
Capable of measuring FHR in the 50-240 beats per minute (bpm) range with 1 bpm resolution and 2 bpm accuracy.
Can monitor twins (i.e., has two transducers).
Includes high/low audible and visual alarms.
Includes signal quality/loss indicators and alarm(s).
Provides audible feedback on signal quality.
MATERNAL UTERINE ACTIVITY MONITORING
Uterine contractions measured though a pressure sensitive transducer.
Capable of measuring relative uterine contractions (UCs) in the range of 0-100 units with at least 1 unit resolution and 1 unit accuracy.
Includes signal loss due to unplugged transducer.
PRINTER/RECORDER
Integrated thermal printer with automatic and manual print-out modes.
Prints FHR1, FHR2, UCs, marked events and parameters and relevant alarms.
Print speeds include 1, 2 and 3 cm/min.
Compatible with z-folded thermal paper.
Two different model are supplied: One model allows for adjusting paper scale between 20 and 30 bmp/cm, the other model has a fixed paper scale of 30 bmp/cm
Includes alarms or another indicator for end of paper.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x Plastic protective dustcover.
1 x UC transducer.
2 x FHR ultrasound transducers.
1 x Remote switch event marker with cable.
3 x Adjustable transducer belts for ultrasound (2 FHRs) and toco (UC).
2 x Box of thermal recording paper, total 100 z-folded sheets.
2 x Bottle of ultrasound gel, approximately 250ml.
OPTIONAL ACCESSORIES AVAILABLE
At extra costs additional optional features can be requested:
- An optional cart can be included.
- An optional back up battery can be included.
ESTIMATED LIFE SPAN
Six years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 5°C – 40°C / 15% - 93% RH.
- Storage conditions: -20°C - 50°C / 15% - 93% RH.
- Atmospheric pressure: 860 ~ 1060 hPa.
- Ingress protection rating Main Unit: IPX0, Probe: IPX1.
WEIGHT AND VOLUME (packaged)
Weight: 13.36 kg.
Volume: 100.00 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Does have specific assembly or commissioning requirements.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS
S0002061 Doppler,FHR detector,w/access.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-2-37:2007+AMD1:2015 CSV Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment.
NOMENCLATURE
- GMDN code: Foetal cardiac monitor (43958).
- UMDNS code: Monitors, Bedside, Foetal, Antepartum (18339)
Related Products