S0002643
Uterine Balloon Tamponade, Sterile, S.U.
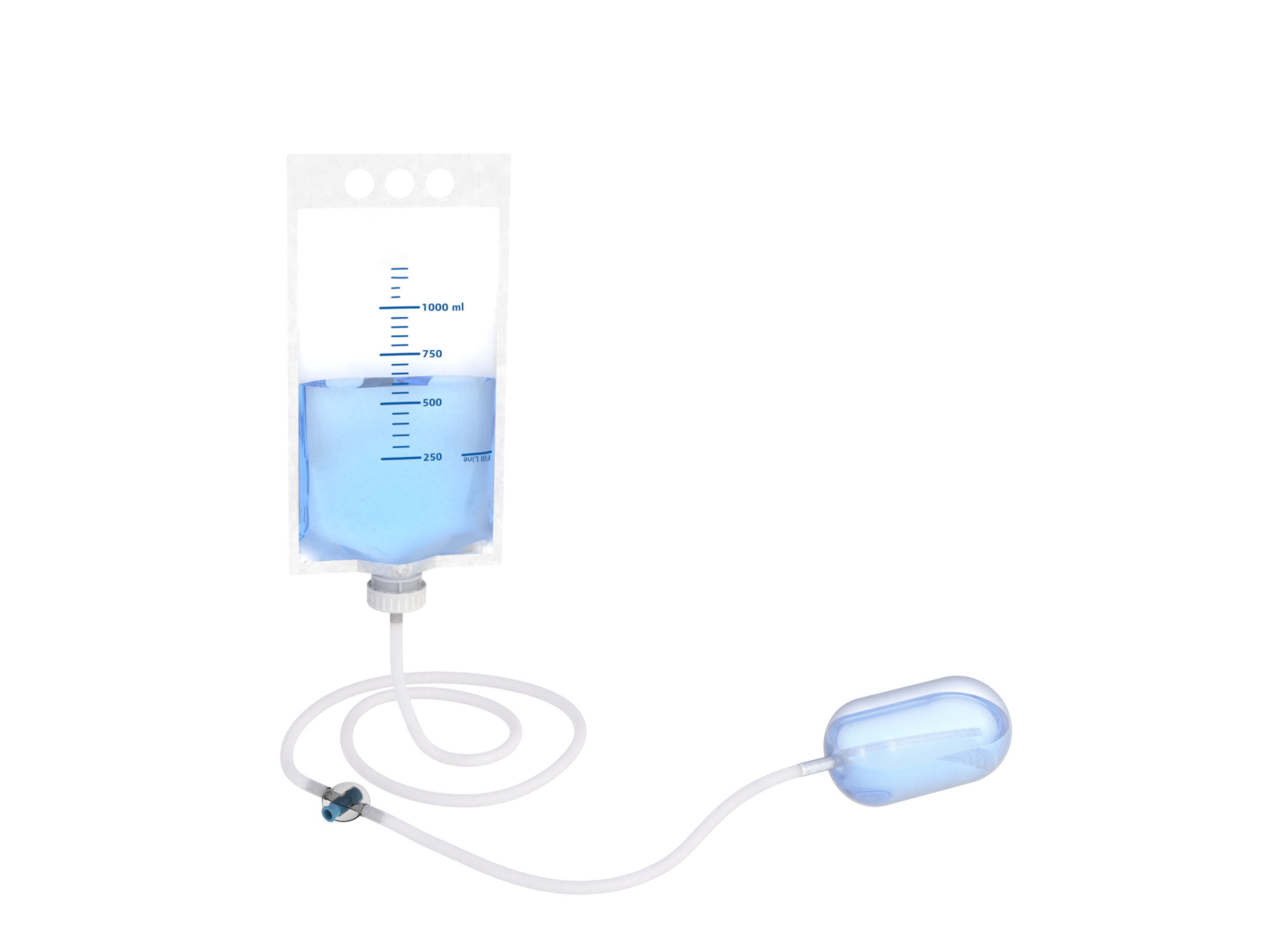
The Uterine Balloon Tamponade (UBT) is a sterile, single-use medical device designed to control postpartum hemorrhage (PPH) by applying pressure to the uterus, thereby reducing blood loss and stabilizing the woman undergoing the condition.
Indicative Price 18.70 USD
INTENDED USE:
Uterine balloon tamponades (UBT) are medical devices that apply pressure to the uterus to reduce blood loss, stabilize the woman undergoing postpartum haemorrhage (PPH), and reduce the need for surgical interventions and blood transfusions. The balloon is inserted into the uterus, filled with saline solution or sterile water, and effective tamponade occurs rapidly to stop the bleeding.
GENERAL DESCRIPTION:
The Uterine Balloon Tamponade (UBT) is a sterile, single-use medical device designed to control postpartum hemorrhage (PPH) by applying pressure to the uterus, thereby reducing blood loss and stabilizing the woman undergoing the condition.
TECHNICAL SPECIFICATIONS:
Material: medical grade elastomer
Capacity: 500 ml – 700 ml filling capacity before balloon stretch
Shape: Diameter 80 mm and cylindrical shape
Thickness: Thin walled for low expansion pressure requirement
Stretch capacity: minimum 2000 ml
Filling mechanism:
The balloon is filled with a syringe or through a gravity-based bag system. The filling needs to be stoppable by a one-way valve, a clamp or a closure tap to ensure pressure control, and there needs to be a quick mechanism to drain the balloon.
Filling time- the time it takes to fill the balloon should be specified by the supplier.
Indwelling time to be specified by the supplier.
No fluid leakage through any part of the device such as the balloon, stopcock etc. during insertion and inflation procedure.
Syringe, if applicable:
Rapid instillation component
Syringe 60 ml
Sterile, for single use
Tube:
Material: Translucent, flexible tube
Length: > 1600 mm
Diameter: > CH22
SHELF LIFE:
3 Years
INSTRUCTIONS FOR USE:
The Uterine Balloon Tamponade (UBT) should be utilized as a secondary method to manage postpartum hemorrhage (PPH) in cases where women do not respond to standard first-line treatments, including the use of uterotonics, tranexamic acid, and intravenous fluids that are available and routinely implemented. It is specifically intended for use when PPH is caused by uterine atony following vaginal birth.
The UBT should only be used in situations where standard protocols for postpartum hemorrhage treatment are available and implemented. It is crucial to consider the possibility of immediate recourse to surgical intervention and access to blood products, if needed. This can be accomplished through referral to a higher-level facility that is prepared for emergencies or, if Comprehensive Emergency Obstetric Care (CemOC) is available, at the point of care.
A detailed Instructions for Use (IFU) document should be provided by the manufacturer, which includes a QR code linking to training and demonstration videos for proper usage.
Warning statements:
·Wherever a uterine balloon tamponade is used, effective maternal analgesia shall be available, if needed.
·To do a visual examination of the vaginal upper third and uterine cervix to exclude trauma-induced bleeding and the placenta to exclude bleeding due to retained placental tissue before employing UBT.
·For use by clinicians who are trained and skilled to treat PPH.
·To continuously monitor the patient after UBT insertion.
CONTRAINDICATIONS:
The use of Uterine Balloon Tamponade (UBT) is contraindicated in the following cases:
1.Traumatic postpartum hemorrhage (PPH)
2.Presence of retained placental tissue
3.Uterine rupture
4.Purulent infection of the vagina, cervix, or uterus
SUPPLIED WITH:
Manufacture's instructions for use in English, French and Spanish.
REGULATION AND CONFORMITY REQUIREMENTS:
CE mark conforming to Medical Device Directive 93/42/EEC Medical Device Directive
CLASSIFICATION:
Classified either under EU MDD 93/42/ECC Class IIa device.
ENVIRONMENTAL CONDITIONS
Storage conditions: 50%RH, 0-55⁰C
Transport conditions: 50%RH, 0-55⁰C
ENVIRONMENTAL REQUIREMENTS:
Not use of PVC polymer
Phthalates free
PACKAGING AND LABELLING:
Primary packaging: Unit of use
One set of Uterine Balloon Tamponade, packaged individually with full instructions in English, French and Spanish.
Secondary packaging:
Individually packed Uterine Balloon Tamponade 25 units per carton
SAFETY & PRODUCT STANDARDS:
Must comply with following standards
MF5 - (EC Declaration of Conformity) Rev 09 - Series of Standards EN ISO 1041:2008+A1:2013, EN ISO 10993-7:2008+A1:2022, EN ISO 11135:2014/A1:2019, EN ISO 11138:2017, EN ISO 11607-1:2020, EN ISO 11737-1:2019/Amd 1:2021, EN ISO 11737-2:2020, EN ISO 13485:2016, EN ISO 14937:2009, EN ISO 14971:2019, ISO 14155: 2020, ISO 15223-1:2021, ISO 20416:2020
WEIGHT AND VOLUME:
Unit weight in KG: 0.24kg
Unit volume in 0.003m3 (CBM)
Related Products