S0002708
Circumcision device,ster,s.u.22mm,PAC-50
Circumcision device sterile disposable for adolescent and adult male 22mm(O) pack of 50
Indicative Price 676.00 USD
GENERAL DESCRIPTION:
Circumcision device sterile disposable for adolescent and adult male 22mm (O) pack of 50
INTENDED USE:
To provide a safe method of performing male circumcision in adolescent and adult populations with the primary goal of reducing the transmission of Human Immunodeficiency Virus (HIV), while also targeting the prevention of Human Papillomavirus (HPV), which is associated with cervical cancer and anal cancer, and Human Herpes Simplex Virus type 2 (HSV-2), which causes genital ulcers and increases the risk of acquiring HIV. This product is intended for use exclusively by trained medical professionals.
The ShangRing comes in a range of 24 sizes (from A4 to Z), each with a unique inside diameter that ranges from 11mm to 40mm.
TECHNICAL SPECIFICATION:
Should be WHO Prequalified Device for Voluntary Medical Male Circumcision for HIV Prevention.
The device is used for male circumcision, defined as the removal or excision of the foreskin or prepuce.
Indicated for use in adolescent and adult males, specifically:
Ages ≥13 years with the flip technique or the no-flip technique.
Ages 10 – 12 years only with the no-flip technique.
The device is available in different sizes to ensure proper fit for individuals of varying ages. The appropriate size for use is determined through the use of a measuring strip, which aids in selecting the correct device size that aligns with the individual's age and anatomical dimensions.
Material: medical grade plastic.
Sterile.
Single use.
Consists of two concentric plastic rings.
The inner ring fits inside the outer ring, which will lock when snapped together.
The inner ring is lined by a silicone pad.
The outer ring is a hinged ring.
The outer ring is composed of two semicircular opening rings.
Hemostasis is achieved by the pressure applied by the interlocking rings, which minimizes bleeding and eliminates the need for sutures.
There is a groove on the outer surface of the inner ring.
The inner side of the outer ring is provided with at least one sharp edge.
The edge and the groove cooperate with each other for pressing and/or cutting the foreskin.
The opening ends of the two semicircular rings link to each other by the junction.
Size selected:
Diameter of inner ring 22 mm (O)
SHELF LIFE:
36 Months
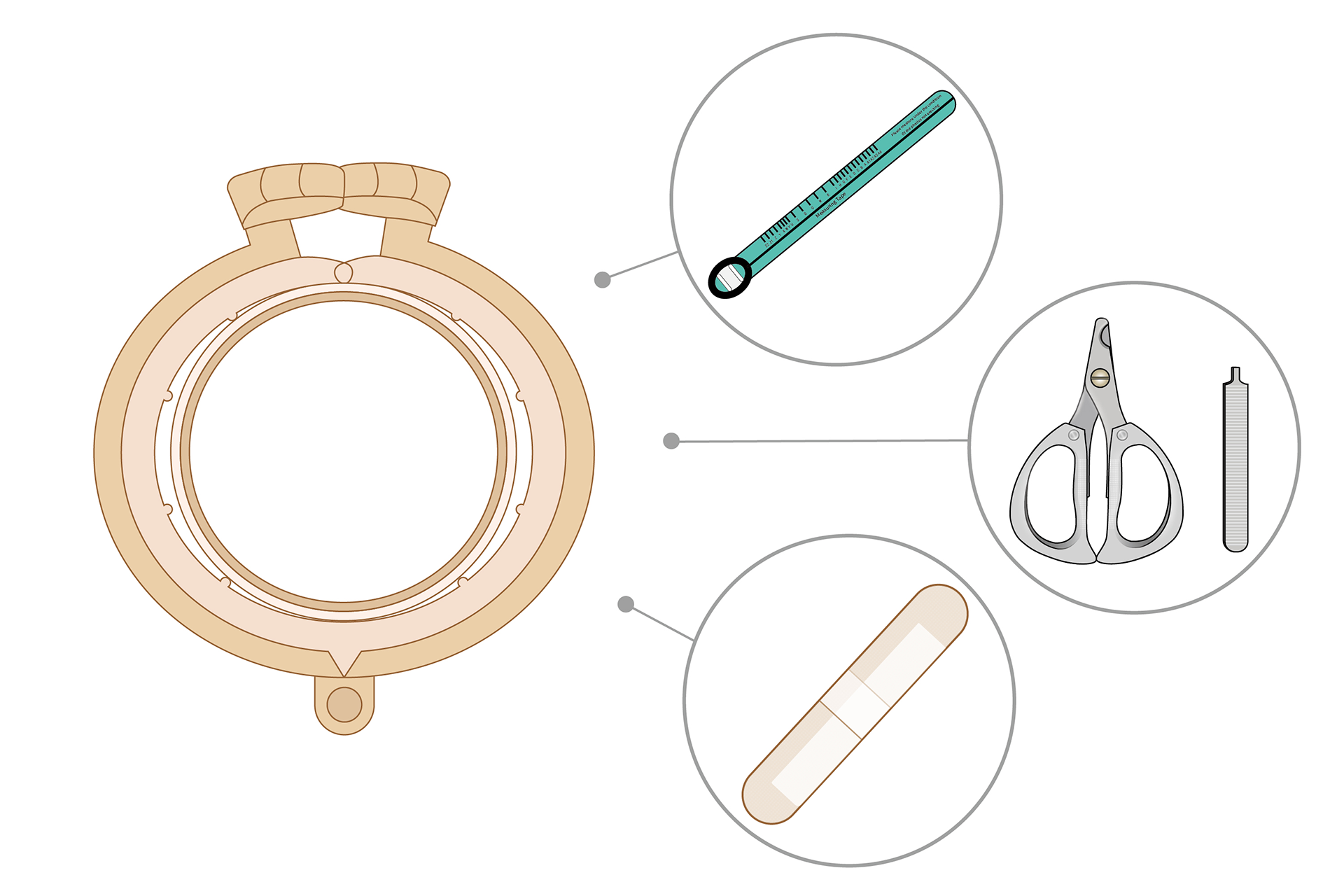
SUPPLIED WITH:
50 sets ShangRing device
50 pcs Measuring tape
350 pcs Bandage
1 set Removal Cutter and Removal Key
Manufacture's instructions for use in English, French and Spanish.
ITEMS REQUIRED, BUT NOT SUPPLIED:
General Hospital Supplies.
Local /Surface Anaesthesia.
Antiseptic solutions use for skin preparation.
Other surgical Instruments.
INSTRUCTIONS FOR USE:
The ShangRing device should be used by healthcare providers, including medical professionals such as medical doctors, nurses, and clinical officers.
For individuals ages ≥13 years, the device can be used with either the flip technique or the no-flip technique.
For individuals ages 10–12 years, the device should only be used with the no-flip technique.
Local anesthesia is necessary for all clients undergoing the circumcision procedure. Injectable anesthesia is the recommended method. However, for individuals without foreskin conditions (phimosis and/or adhesions), topically applied EMLA cream may be considered as an alternative to injectable anesthesia, but only when using the no-flip technique.
REGULATION AND CONFORMITY REQUIREMENTS:
CE mark, demonstrating conformity to either Medical Device Directive 93/42/EEC (or Medical Devices Regulation (EU) 2017/745).
Compliance with applicable regulations from FDA (U.S.), Australia TGA (Therapeutic Goods Administration),
Canada MDL (Medical Device Licence).
ISO 13485 certification, indicating compliance with the international standard for quality management systems specific to medical devices.
CLASSIFICATION:
Under EU regulations: It is classified as either Class IIa or higher device, categorized under EU MDD 93/42/EEC, or under EU MDR 2017/745.
Under Australia TGA regulations: It is classified as a Medical Device Class IIa or higher.
Under Canada MDL regulations: It is classified as a Device Class/Classe de l'instrument 2 or higher.
ENVIRONMENTAL CONDITIONS
Storage conditions: 5℃ to 50℃, humidity up to 80%.
Transport conditions: 5℃ to 50℃, humidity up to 80%.
ENVIRONMENTAL REQUIREMENTS:
Not use of PVC polymer
Phthalates free
PACKAGING AND LABELLING:
Primary packaging: Unit of use
One set of individually packed sterile, single-use, male circumcision devices with an inner diameter of 22 mm in total 50 packs with accessories.
With full instructions in English, French and Spanish.
Secondary packing:
LABELLING:
In accordance with UNICEF technical requirements as indicated here
Additionally labelling in accordance with EU MDD 93/42/EEC, Annex I, point 13.3
or
In accordance with UNICEF technical requirements as indicated here
Additionally, labelling in accordance with EU MDR 2017/745 Annex I, Chapter III, point 23.2 for details on this regulation.
SAFETY & PRODUCT STANDARDS:
Must comply with following standards
ISO 13485:2016 Medical devices -Quality management systems -- Requirements for regulatory purposes.
ISO 14971:2007 Medical Devices- Application of risk management to medical devices.
EN ISO 15223-1 (EN 980) Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements.
Or equivalent international standard.
WEIGHT AND VOLUME:
Unit weight in KG: 4.05
Unit volume in CBM (M3) or CDM (DM3): 0.03 m3
Related Products