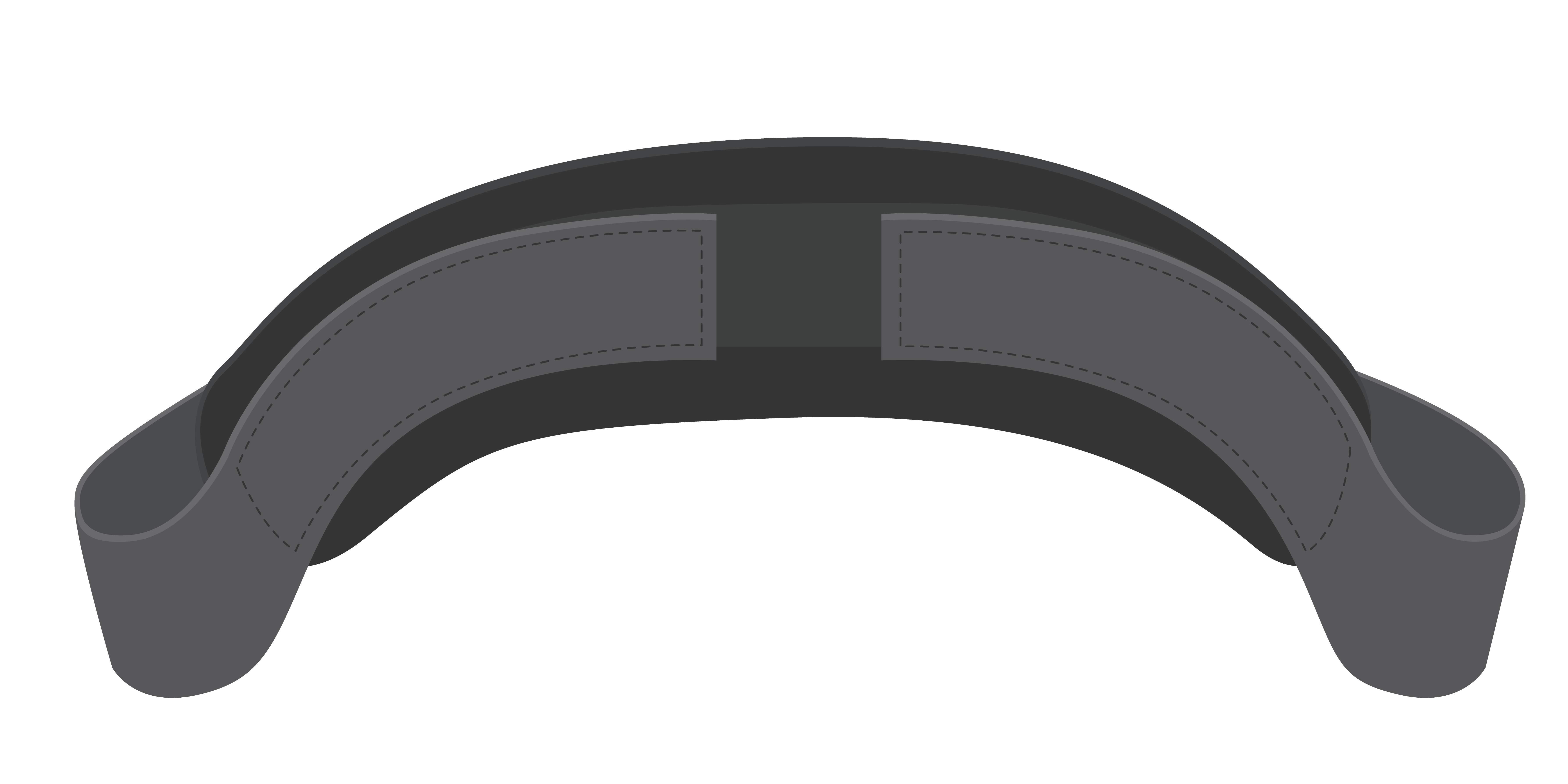
S0004021
Strap lower leg/calf, wheelchair
Calf strap:
FUNCTION AND POSTURE SUPPORT: For CHILD and ADULT wheelchair users to optimise posture support of the lower legs and feet. Prevents the feet from sliding off the back of the foot support/s. Durable, adjustable straps with universal design to be fitted to a range of wheelchairs.
ENVIRONMENT: For indoor and outdoor use.
Indicative Price 19.50 USD
GENERAL DESCRIPTION
Calf strap for wheelchair
IMPORTANT INFORMATION
Minimum order quantities may apply depending on the model selected.
Inappropriate prescription and fitting of wheelchairs, cushions and accessories may harm the user’s health and/or function. Therefore, this product should only be purchased after consultation with the Medical Devices Unit in Supply Division.
INTENDED USE
Calf strap:
FUNCTION AND POSTURE SUPPORT: For CHILD and ADULT wheelchair users to optimise posture support of the lower legs and feet. Prevents the feet from sliding off the back of the foot support/s. Durable, adjustable straps with universal design to be fitted to a range of wheelchairs.
ENVIRONMENT: For indoor and outdoor use.
TECHNICAL SPECIFICATIONS
One-piece unit
Attach securely to lower leg support frame and accommodate different diameters of frame.
Length adjustable to optimise fit after fastened
Padded in calf area (depending on model selected)
Fastener: user-friendly, quick-release, hook-and-loop fasteners
Skin protection through:
- Contours to the body
- Design to prevent curling of edges, bunching, wrinkling, deforming or fraying and/or localised pressure points
Washable
No dedicated mounting mechanism. Wraps around frame of lower leg support of wheelchair.
Size and dimensions:
Depending on model selected: 5 or 6 sizes to fit wheelchair seat width range 250-500 mm.
50-130 mm wide depending on model and size selected
ASSEMBLY
Fully assembled
SUPPLIED WITH:
Detailed illustrated instructions to mount/attach to wheelchair
Detailed illustrated instructions for safe use
WARRANTY
Limited warranty to cover defects in material and workmanship only. Warranty excludes normal wear or damage following inappropriate use or lack of maintenance.
Warranty: 1 OR 2 years depending on model selected
MINIMUM ORDER QUANTITIES
Minimum order quantities may apply depending on model selected
PACKAGING, LABELLING, INSTRUCTIONS:
Please note: Minimum order quantities may apply depending on model selected
One (1) unit per box
Dimensions:
- Unit Weight in Kg (including its packaging): 0.5 kg
- Unit Volume in M3 (including its packaging): 0.01 m³
Labelling: Compliance with EAN 128 bar code requirements
ESTIMATED LEAD TIME
60 days
ENVIRONMENTAL CONDITIONS
Operating conditions: 0 to 50 degrees Celsius; 20-60% RH
Storage conditions: 0 to 50 degrees Celsius; 10-85% RH
ESTIMATED LIFE SPAN
1-5 years depending on model selected
USER FITTING AND TRAINING REQUIREMENTS
Users to be fitted and trained by trained wheelchair service personnel.
MAINTENANCE REQUIREMENTS
Product maintenance as per manufacturer’s instructions
RELATED PRODUCTS
S0004004 Wheelchair, assistnt controlld,transport
S0004005 Wheelchair,active urban,fold,more support
S0004006 Wheelchair, active urban, folding
S0004007 Wheelchair, active urban, rigid
S0004008 Wheelchair, active dual terrain, folding
S0004009 Wheelchair, active dual terrain, rigid
S0004010 Wheelchair, active rough terrain
S0004011 Wheelchair, postural support
S0004012 Cushion,wheelchair, posture, layered
S0004014 Cushion,wheelchair,pressure, mod layered
S0004015 Cushion,wheelchair,pressure,mod no layer
S0004018 Cushion,wheelchair,pressure,deep,section
ALTERNATIVE PRODUCTS
S0004020, Strap pelvis/hip, wheelchair
S0004022 Strap ankle/foot, wheelchair
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDR 2017/745 as Class I device.
NOMENCLATURE
GMDN Code:
13356
Related Products