S0305160
Respirator,FFP2/Surg N95,fluid-resist
FFP2, N95, respirator, surgical, fluid resistant
Indicative Price 0.71 USD
General Description:
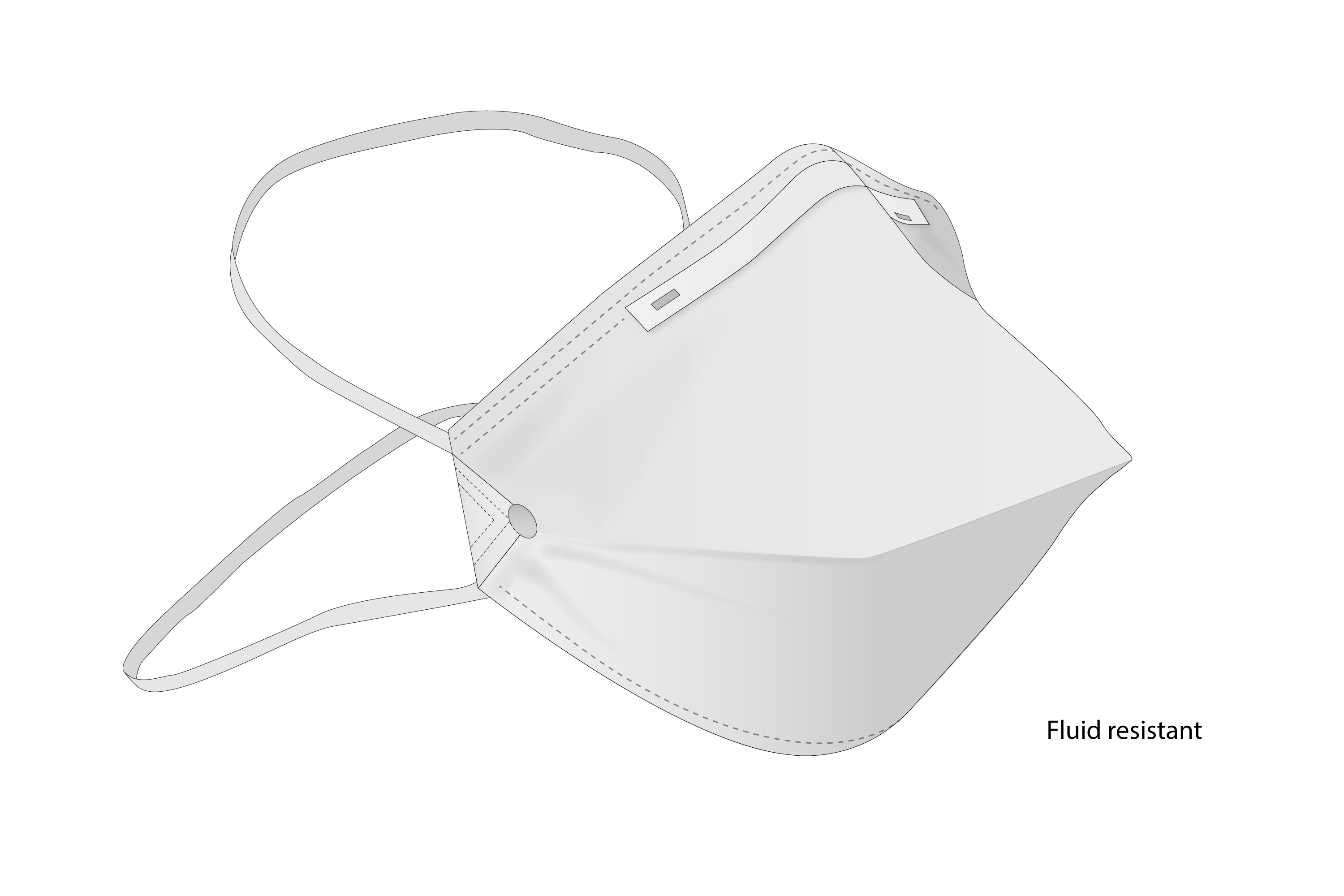
Fluid resistant respirator protecting against airborne pathogens.
Intended use:
To reduce the healthcare workers risk of inhaling hazardous airborne particles (including dust particles and infectious agents), gases, or vapour. Worn on the face and covers at least the nose and mouth.
Technical specifications
Material: non-woven filter layer
Good particle filtration (minimum 94% or 95%) for particles from 0.1 to 0.3 micron
Air permeability: > 2 mm H2O
Shape of the respirator: duckbill, folded (horizontal) width-wise
Without valve
Splash resistant
Respirator fits all face shapes, without inspiration/expiration air-leakage
Upper edge has integrated easy malleable nose bridge strip reducing fogging of protective eye-wear
Size nose bridge strip: 4 x 90 mm (w x l) (+/-10%)
Two pre-attached elasticated straps, fitting (i) around top of the head ,(ii) around base of the head
Colour: white
Non-sterile
Single use, disposable
Each respirator bares clear identification of
(i) protection provided FFP2/N95
(ii) which side to wear up (nose),
(iii) manufacturer's name, and
(iv) model reference
Fluid resistant respirator conforms to standards:
NIOSH N95 recommended by the CDC and WHO for healthcare workers. The disposable respirator should be marked with the manufacturer's name, the part number (P/N), the protection provided by the filter (e.g. N-95) , and NIOSH 42 CFR 84, FDA minimum "surgical N95"
Or
EN 149, minimum "FFP2" and EN 14683 Type IIR. The disposable respirator should be marked with the manufacturer's name, standards and CE certification number (European Union (EU) Module C2 or D conformity to type certificate (Category III PPE). Regulation (EU) 2016/425 on personal protective equipment Category III.
Or
or equivalent international standard
Supplied with: Supplier's instruction for use
Packaging and labelling:
Primary packaging: Unit of use
One (1) piece
Labelling of the primary packaging displays, at least: product name , product reference, manufacturer name, size, type and performance testing information against the mentioned standards. Information for particular storage conditions (temperature, pressure, light, humidity, etc.), as appropriate (or equivalent harmonised symbol), if applicable. All indicated at least in English.
Related Products