S0323304
Catheter,Foley,CH16,ster,disp
Catheter,Foley,CH16,sterile,disposable
Indicative Price 0.36 USD
GENERAL DESCRIPTION:
Catheter,Foley,CH16,ster,disp
DEFINITION:
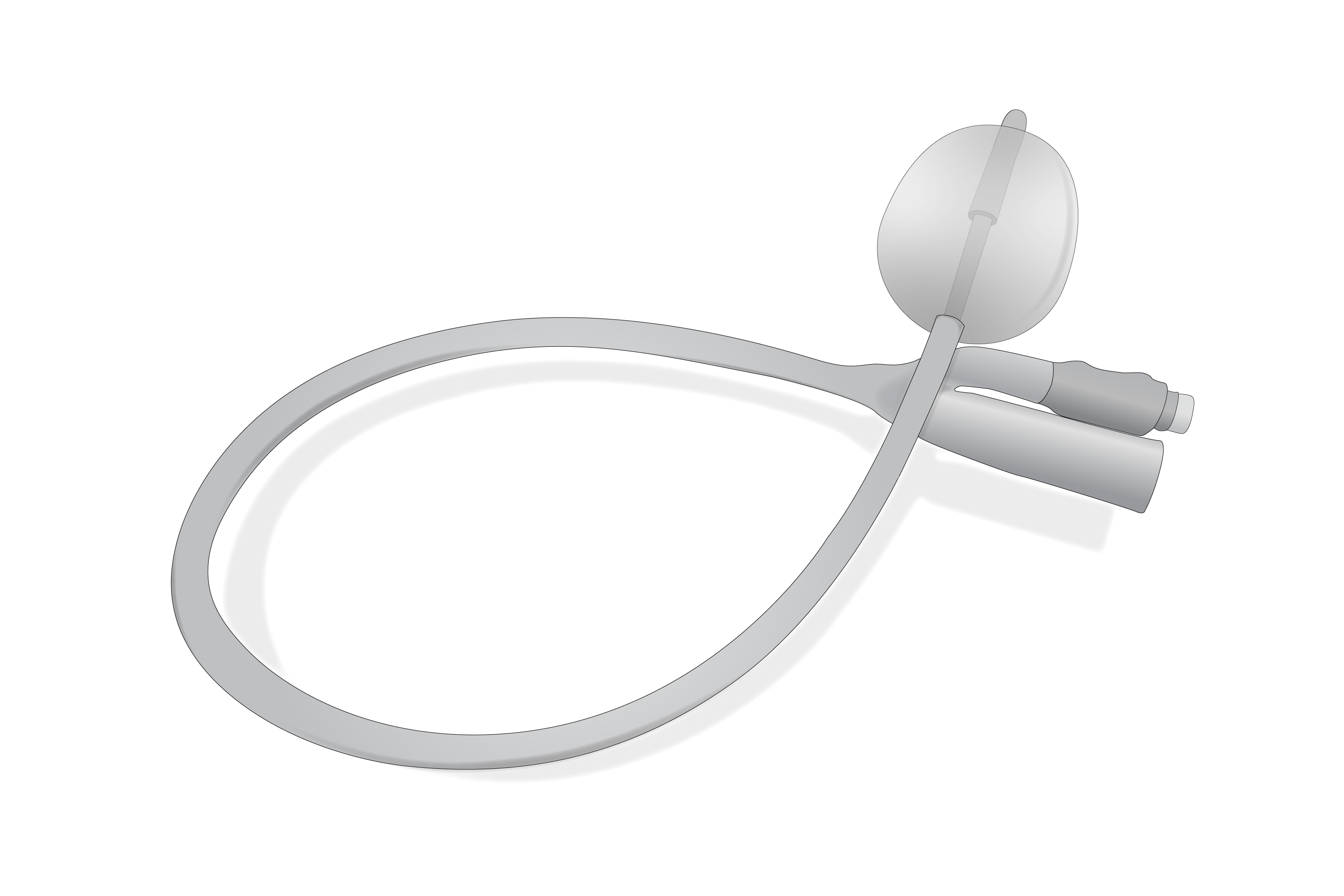
A sterile, flexible tube with an inflatable balloon at its distal tip intended for insertion through the urethra and retention in the urinary bladder for indwelling therapeutic urinary drainage. Designed for single-use only, it serves male and female patients and must be introduced by a healthcare professional.
TECHNICAL SPECIFICATIONS
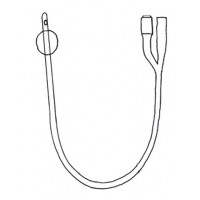
Urethral catheter with balloon, Foley catheter 2-way with Nelaton tip.
Central channel for urinary drainage, straight rounded distal end with lateral eyes, proximal end with cup connector for urine bag connection.
Side channel for inflating balloon, ending with a non-return valve with Luer tip connector.
Diameter expressed in Charriere, French gauge.
Length expressed in cm.
Balloon capacity expressed in ml.
Color code / external diameter visible on connector.
Material: Natural latex, silicone-coated.
Single-use.
Sterile.
Initial sterilization method: Ethylene Oxide gas or Gamma Radiation. Indicate the sterilization method
DIMENSIONS:
Diameter: CH16
Length: 30 - 45cm
Balloon capacity: 5 -15ml
SHELF LIFE: 3 or 5 years depending on the supplier.
PACKAGING:
Primary packaging: Unit of use: One (1) Foley catheter in an labeled sterile peel-open pack.
Secondary packaging: Protected unit.
One (1) box of 10 Foley catheters.
Manufacturer's instruction for use. Alternatively, the instruction for use can be indicated on a separate insert.
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
Supplier is certified for ISO 9001 Quality management systems – as a minimum requirement.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDR 2017/745 as Class IIa device or higher.
SAFETY AND PRODUCT STANDARDS
Comply with the following standards:
EN ISO 20696:2018 – Sterile urethral catheters for single use.
EN ISO 13485:2016/A11:2021 – Quality management systems for medical devices.
EN ISO 14971:2019 – Application of risk management to medical devices.
EN ISO 11135:2014/A1:2019 – Sterilization of health care products using ethylene oxide.
EN ISO 11607-1:2020 – Packaging for terminally sterilized medical devices (Part 1).
EN ISO 11607-2:2020 – Packaging for terminally sterilized medical devices (Part 2).
EN ISO 15223-1:2021 – Symbols to be used with medical device labeling.
EN ISO 20417:2021 – Information to be provided by the manufacturer.
NOMENCLATURE
GMDN Code:
COMPONENT OF A KIT: Yes, S9901050 IEHK 2024,Suppl. Renewable UNIT
Related Products