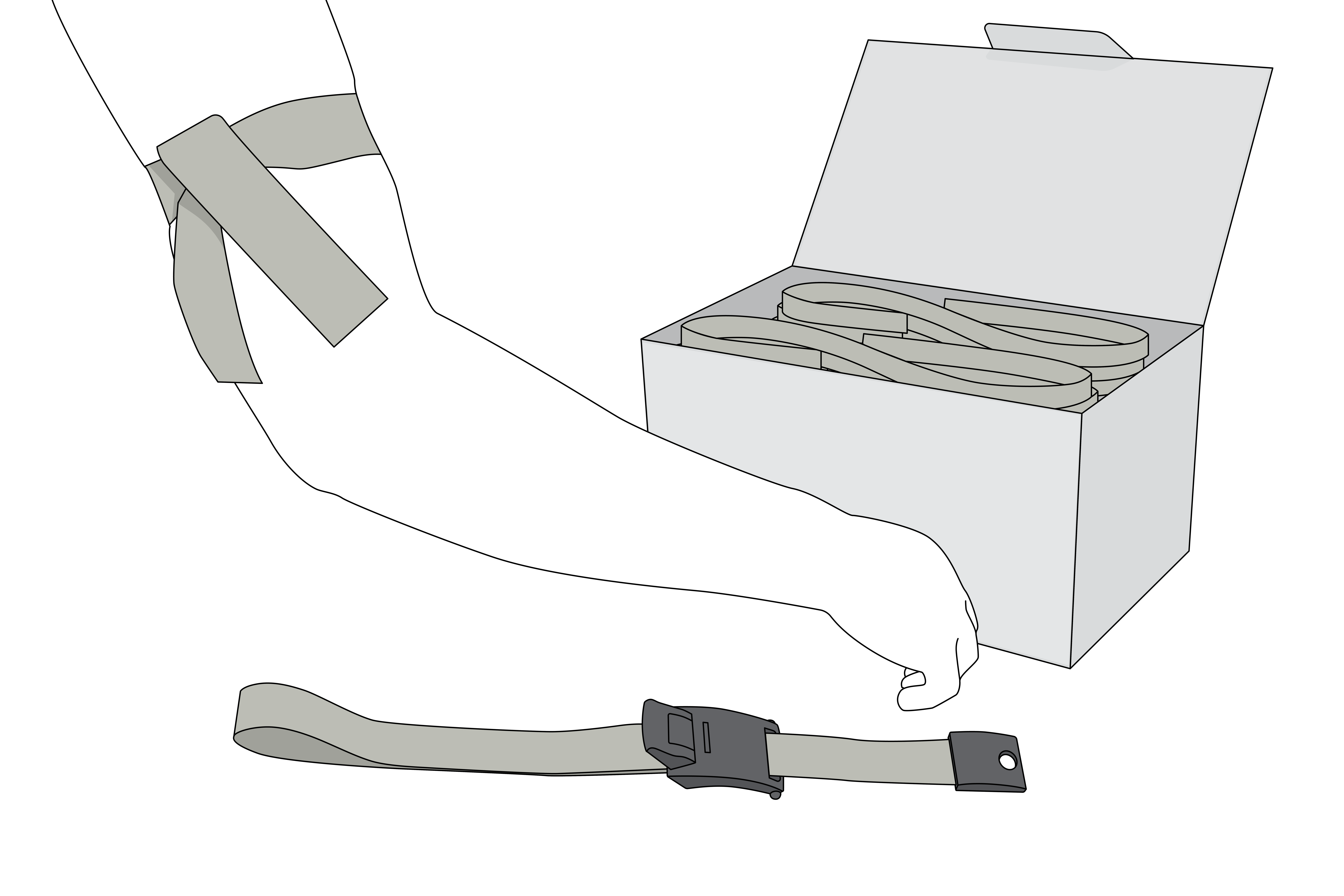
S0385010
Tourniquet,latex-free,50cm
Elastic tourniquet with a quick release buckle.
Indicative Price 2.73 USD
GENERAL DESCRIPTION
Tourniquet, with a buckle.
INTENDED USE
A strap intended to be wrapped around a patient's limb (arm or leg) and manually tightened to reduce blood circulation to or from the portion of the limb distal to where the device is applied. It is typically used by emergency medical services (EMS) for emergency blood loss prevention or for routine blood sampling.
TECHNICAL SPECIFICATIONS
Tourniquet used to compress a limb to stop blood flow.
An elastic band with a quick release buckle.
Material: Latex free.
Width: 2.3 - 2.5 cm
Length: 40 - 56 cm
Reusable, non-sterile
Can withstand hospital grade disinfection agents
Autoclavable at 100°C.
SHELF LIFE
Not applicable.
ESTIMATED LIFE SPAN
Ten years or 10.000 uses, depending on the model supplied.
WARRANTY
Two or three years depending on the model supplied.
ENVIRONMENTAL CONDITIONS
Storage conditions: 0 - 50°C / 85% RH.
Operating conditions: 10 - 40°C / 85% RH.
WEIGHT AND VOLUME (Packaged)
Weight: 55 gr.
Volume: 0.2 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
No installation or commissioning requirements.
TRAINING REQUIREMENTS
No user training will be required.
MAINTENANCE/USER REQUIREMENTS
No maintenance requirements.
COMPONENT OF A KIT
S9901029 - IEHK2017,kit,suppl.2-equipment
S9903002 - AWD, Periphery kit, Equipment
S9902221 - Midwifery kit – equipment, 2
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDR 2017/745 as Class I device.
NOMENCLATURE
GMDN Code: 58128
Related Products