S0531990
Infusion giving set,w/burette,ster,s.u.
Infusion giving set, with burette, sterile, single use
Indicative Price 0.60 USD
General Description:
Infusion giving set, with burette, sterile, single use
Technical Specifications:
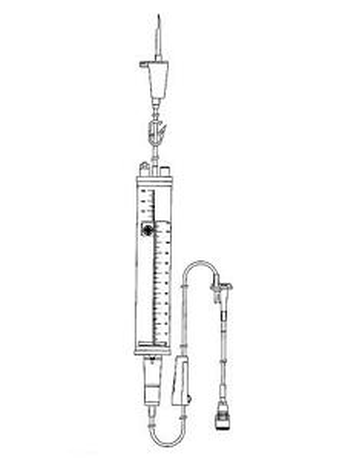
Sterile IV giving set with graduated chamber (burette) enabling precise volume and slow administration of infusion or injectable medicine.
Tube: plastic (PVC: polyvinyl chloride). Transparent (allowing the detection of air bubbles). Resistant to kinking.
Length is approximately 150cm (overall IV giving set length approximately 170cm). Internal / external diameter is approximately 3mm / 4mm.
Plastic Perforator: plastic (polyacetate).
Hollow device located at the proximal end of the infusion set allowing it to be connected to the infusion bottle or bag. Composed of a tapering tube mounted on a base ensuring a good seal between the set and the infusion bottle or bag.
Fitted with a protecting cap.
Air inlet: plastic (ABS: acrylonitrile butadiene styrene)
Incorporated into the perforator. Fitted with an air filter (bacteriological filter).
Graduated chamber (burette): plastic (PS: polystyrene).
Transparent. Capacity: approximately 100-150ml. Graduations every ml. Connected to the perforator by a tube (approximatey 15cm).
Security gauge:
Automatic shut off valve, to stop the infusion as soon as the pre-set volume has flown away in order to prevent air entering the drop-counting chamber and/or the tube.
Drop-counting chamber:
Located under the graduated chamber. Calibrated 60 drops/ml. Fitted with a 15µ polyamide filter.
Flow regulator: plastic (ABS: acrylonitrile butadiene styrene).
Ensures the flow regularity during the IV administration.
Injection portal: plastic (ABS: acrylonitrile butadiene styrene)+ synthetic rubber.
Allows introducing precise volume of injectable medicines into the infusion flow.
Terminal connection (stopper): plastic (ABS: acrylonitrile butadiene styrene).
With Luer lock connector.
Single use.
Sterile.
Packaging and labelling:
Primary packaging: Unit of use.
One (1) Infusion giving set in an individual sterilised peel pack.
Secondary packaging: Protected unit.
One (1) box of X Infusion giving set units.
Over packaging: packaging unit
Accessories/Spare parts/Consumables: N
Weight/Volume/Dimensions:
estimated weight: 0.195kg
estimated volume: 1.06cdm
Instructions for use:
Sterile infusion giving set with graduated chamber enabling a slow intravenous administration of a precise volume of infusion or injectable drug over a given time. It is to be connected between the infusion and the intravenous catheter.
An infusion giving set should not be left in situ for more than 72 hours.
Storage conditions:
Avoid storage at extreme temperatures and humidity levels
Safety process:
The sterile infusion giving set is for single use only.
Follow the rules of asepsis when using it.
After use, dispose the infusion giving sets into a safety waste container. It should be collected and destroyed by incinerating in controlled surroundings or disposed of in a safe burial pit in compliance with national laws and regulation on health care waste management.
For more information on waste management, please refer to WHO publication "Safe Management of Wastes from Health Care Activities".
Component of a kit: N
Related Products