S0738000
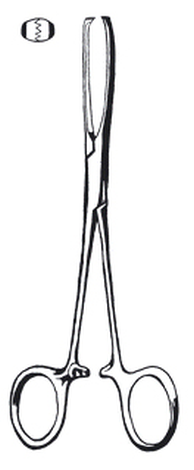
Forceps,tissue,Allis,150mm
Forceps, tissue seizing, Allis, 150 mm.
Indicative Price 2.16 USD
General Description:
Forceps, tissue seizing, Allis, 150 mm.
Intended use:
For grasping organs, soft or heavy tissue.
Technical Specifications:
Pressure force instrument: tissue grasping forceps, springy.
See Martin Catalogue no. 24 Ref: 30 132 15.
4 x 5 teeth.
Precise adjustment of the teeth.
Hard ratchet, lockable.
Length: 15 cm.
Material: Martensitic steel (quenched, magnetic steel).
Martensitic steel composition: 0.15-0.20% carbon, 12-13% chromium.
Reusable.
Environmental conditions:
Storage and transport conditions: 1°C to 30°C.
Humidity: 30% RH to 70% RH.
Quality Management System:
ISO 13485:2016: Medical devices - Quality management systems
Classification:
EU Medical Devices Directive 93/42/ECC, Class I
Compliance to safety & product performance standards:
ISO 7153-1:2016: Surgical instruments - Materials - Part 1: Metals.
ISO 7151:1988: Surgical instruments - Non-cutting, articulated instruments - General requirements and test methods.
ISO 13402:1995: Surgical and dental hand instruments - Determination of resistance against autoclaving, corrosion and thermal exposure.
ISO 17664:2017: Processing of health care products - Information to be provided by the medical device manufacturer for the processing of medical devices.
ISO 14971:2007 Medical devices - Application of risk management to medical devices.
Nomenclature:
GMDN code: Surgical soft-tissue manipulation forceps, scissors-like, reusable (62468)
UMDNS code: Forceps, Tissue (11797)
Safety process:
This item must be cleaned, disinfected after each use, and sterilised in a steam steriliser.
Packaging and labelling:
Primary packaging: Unit of use.
One (1) forceps in a plastic bag.
Secondary packaging: Protected unit.
Ten (10) forceps in a box.
Labelling on the primary packaging:
Name and/or trademark of the manufacturer.
Manufacturer's product reference.
Type of product and main characteristics.
Information for particular storage conditions (temperature, pressure , light, humidity, etc.), as appropriate (or equivalent harmonised symbol).
Information for handling, if applicable(or equivalent harmonised symbol).
Over packaging: Packaging unit
Labelling on the packaging unit: Labelling to be the same as primary packaging.
Extra information required: Number of units per box.
Any symbol used on the labelling should be in accordance of the ISO 15223-1: 2016:
If the item has a CE marking (or equivalent), the CE logo (or equivalent) must be visible on the packaging.
Component of a kit:
S9910000 - Surg. inst., abdominal /SET.
S9910001 - Surg. inst., basic surgery /SET
Related Products