S0778000
Speculum,vaginal,Graves,115x35mm
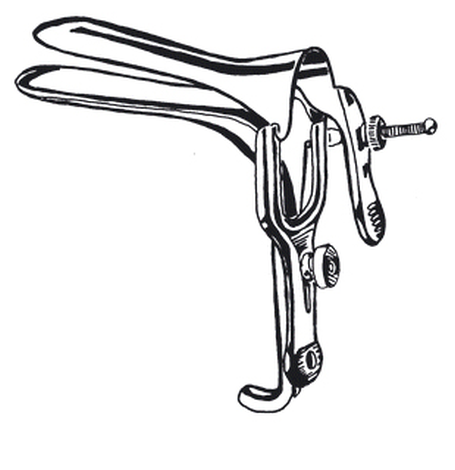
Speculum, vaginal, Graves, 115 x 35 mm.
Indicative Price 3.51 USD
General Description:
Speculum, vaginal, Graves, 115 x 35 mm.
Intended use:
To dilate the vagina to examine the vaginal canal and cervix.
Technical Specifications:
Speculum, vaginal, self-retaining.
See Martin Catalogue no. 24 Ref: 32 130 03.
Double blade with a screw mechanism to keep the blades open.
Blade length: approx. 115 mm.
Blade width: approx. 35 mm.
Material: Austenitic steel (non-quenched, non-magnetic steel).
Austenitic steel composition: 16 - 18% chromium; 10 - 14% nickel; 2 - 3% molybdenum.
Reusable.
Environmental conditions:
Storage and transport conditions: 1°C to 30°C
Humidity: 30% RH to 70% RH
Quality Management System:
ISO 13485:2016: Medical devices - Quality management systems
Classification:
EU Medical Devices Directive 93/42/ECC, Class I
Compliance to safety & product performance standards:
ISO 7153-1:2016: Surgical instruments - Materials - Part 1: Metals.
ISO 7151:1988: Surgical instruments - Non-cutting, articulated instruments - General requirements and test methods.
ISO 13402:1995: Surgical and dental hand instruments - Determination of resistance against autoclaving, corrosion and thermal exposure.
ISO 17664:2017: Processing of health care products - Information to be provided by the medical device manufacturer for the processing of medical devices.
ISO 14971:2007 Medical devices - Application of risk management to medical devices.
Nomenclature:
GMDN code: Vaginal speculum, reusable (35352)
UMDNS code: Specula, Vaginal (13666)
Safety process:
This item must be cleaned, disinfected after each use, and sterilised in a steam steriliser.
Packaging and labelling:
Primary packaging: Unit of use
One (1) speculum in a plastic bag.
Secondary packaging: Protected unit.
Ten (10) speculums in a box.
Labelling on the primary packaging:
Name and/or trademark of the manufacturer.
Manufacturer's product reference.
Type of product and main characteristics.
Information for particular storage conditions (temperature, pressure , light, humidity, etc.), as appropriate (or equivalent harmonised symbol).
Information for handling, if applicable (or equivalent harmonised symbol).
Over packaging: Packaging unit
Labelling on the packaging unit: Labelling to be the same as primary packaging.
Extra information required: Number of units per box.
Any symbol used on the labelling should be in accordance of the ISO 15223- 1: 2016:
If the item has a CE marking (or equivalent), the CE logo (or equivalent) must be visible on the packaging.
Component of a kit:
S9910006 - Surg. inst., exam/sut. vaginal/cervical /SET.
Related Products