S0782403
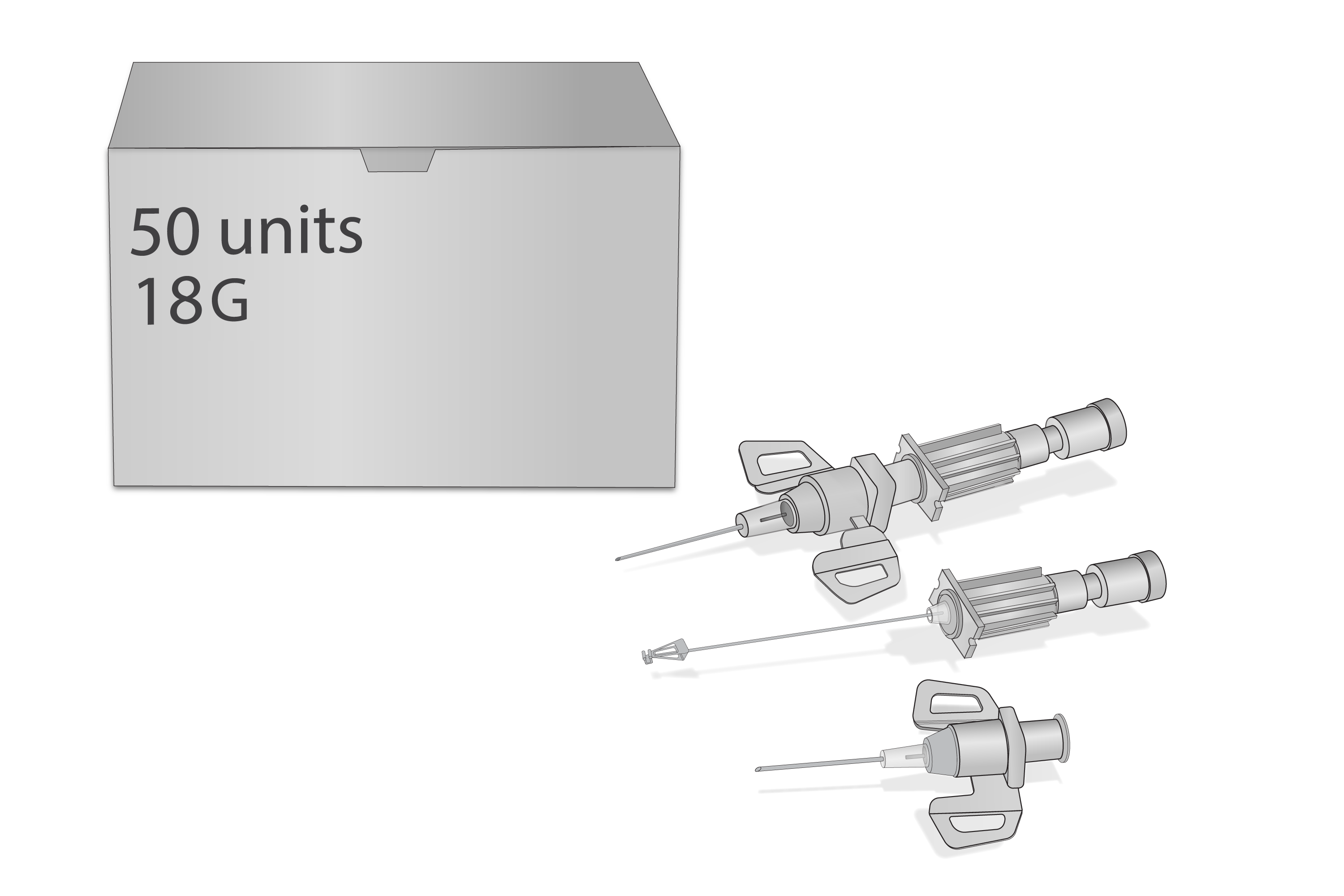
Cannula,IV short,18G,SIP,ster,disp,BOX50
Cannula, IV short, 18G, SIP, sterile, disposable
Indicative Price 12.00 USD
General description: Cannula, IV short, 18G, SIP, sterile, disposable
Technical specifications:
Components: Protecting cap, trocar, cannula, stopper, injection port Luer lock, sharps injury prevention feature conform to ISO 23908:2013 to protect against accidental needle stick injuries (all parts fit together to form a unit).
External diameter expressed in Gauge and mm.
Length expressed in mm.
Colour code/external diameter: Visible at the base of cannula.
Components: Protecting cap, trocar, cannula, stopper, injection port Luer lock (all parts fit together to form a unit).
Material:
Trocar: stainless steel.
Cannula: PTFE (Poly Tetra Fluoro Ethylene), FEP (Fluorinated Ethylene Propylene), PUR (Polyurethane).
Size selected: 18G (1.30 x 45mm) green.
Disposable.
Sterile.
Initial sterilization method: Ethylene oxide gas.
Shelf life:
60 Months
Packaging and labelling:
Primary packaging: Unit of use.
One (1) IV cannula in an individual sterilised peel pack.
Secondary packaging: Protected unit.
One (1) box of 50 IV cannulas.
Instructions for use:
Sterile IV cannula to be introduced into a peripheral vein for infusing fluids, administering IV drugs.
The selected sizes are the most commonly used:
- 16G (1.70 x 45mm) grey, flow rate approx. 180ml/min, for adults: IV infusion.
- 18G (1.30 X 45mm) green, flow rate approx. 80ml/min, for adults: IV infusion.
- 20G (1.00 X 32mm) pink, flow rate approx. 55ml/min, for adults and infants: IV infusion.
- 22G (0.90 X 25mm) blue, flow rate approx. 33ml/min, for infants and children: IV infusion.
- 24G (0.70 X 19mm) yellow, flow rate approx. 20ml/min, for children and neonate: IV infusion.
Conditions for stock:
Avoid storage at extreme temperatures and humidity levels.
Check the integrity of each unit before use.
Single use material, supplied in sterile packaging, may only be used if the packaging is undamaged.
Safety process:
IV cannula is for single use only.
Rules of asepsis must be followed when inserting IV cannula.
IV cannula should not be left in situ for more than 72 hours. It should be removed immediately in case of infection sign.
Protection of users:
To prevent stick injuries, wear gloves.
NEVER recap the trocar after use and dispose of the needle into a puncture proof safety container.
Safety boxes are available as a UNICEF standard warehouse item reference: S0782208 - Safety box f.used syrgs/ndls 5lt/BOX-25.
Collect and destroy puncture proof containers either by incinerating them in controlled surroundings or dispose of them in a safe burial pit in compliance with national laws and regulation on health care waste management.
For more information on waste management, please refer to WHO publication "Safe Management of Wastes from Health Care Activities".
Weight and volume
Estimated weight: 0.0035kg
Estimated volume: 0.0165cdm
Component of a KIT:
S9908302 - Obstetric, surgical kit, suppl.3-renewable
S9906627 - IEHK2006, kit, suppl.3-renewable
S9906730 - Diarrhoeal Disease Set Packing
Related Products