S0845156
Ophthalmoscope,set
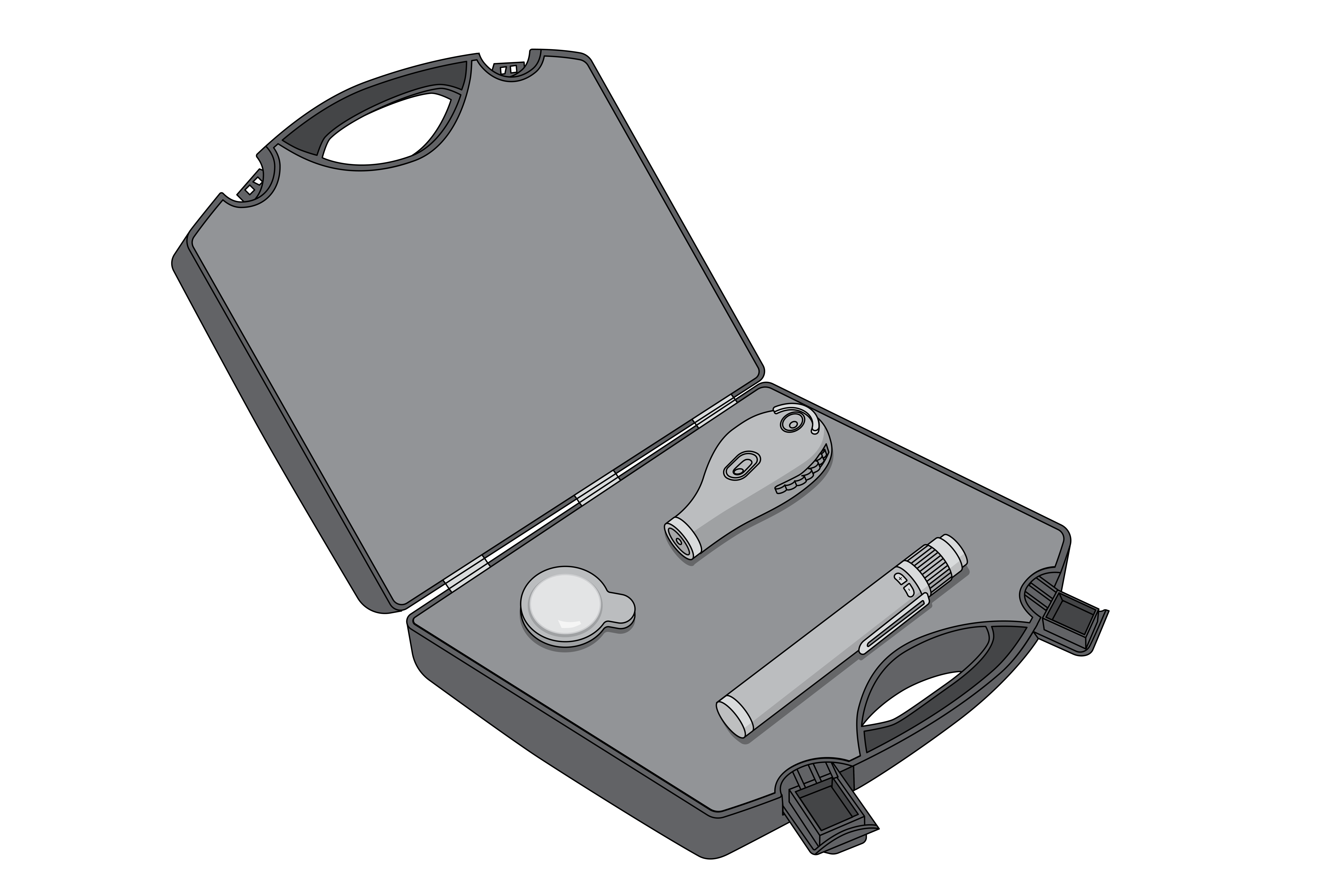
Ophthalmoscope set to visualize the interior of the eye, with the instrument relatively close to the subject's eye and the observer viewing an upright magnified image. Ophthalmoscope set composed of diagnostic head threaded on a handle.
Indicative Price 82.02 USD
GENERAL DESCRIPTION
Ophthalmoscope including accessories.
INTENDED USE
An electrically-powered, hand-held, ophthalmic instrument designed to be held close to the patient’s eye to examine the interior of the eye and related structures [e.g., fundus (retina), cornea, aqueous, lens, and vitreous] by producing an upright (unreversed) image of approximately 5 times magnification. It consists of a main unit (commonly referred to as the head) with a built-in light source directed through the pupil to illuminate the interior of the eyeball, a mirror with a single hole for viewing, and a dial containing lenses of different powers; a dedicated power supply handpiece may be included with the head.
TECHNICAL SPECIFICATIONS
Ophthalmoscope set composed of diagnostic head mounted on a handle.
Range of lenses not smaller than -35D to +40D, adjustable in steps
Anti-reflection lens.
Ability to magnify at least five times.
Available apertures at a minimum: small, large and semi-circle, fixation star.
Colour temperature: Cool white in the range 3,500-4,000 K.
Red-free, blue, and green filters.
Handle with on/off switch.
Provided with an LED light source.
Is able to withstand frequent cleaning and disinfecting with hospital grade products.
SUPPLIED WITH
1 x plastic protective hard case or strong protective pouch containing full set.
1 x set of suitable disposable alkaline batteries.
ESTIMATED LIFE SPAN
Between five to ten years depending on the supplied model.
WARRANTY
Two years.
ENVIRONMENTAL CONDITIONS
Storage conditions: 0 - 50°C / 85% RH.
Operating conditions: 10 - 40°C / 85% RH.
Atmospheric pressure: 800 hPa – 1060 hPa.
WEIGHT AND VOLUME (Packaged)
Weight: 647 gr.
Volume: 1.5 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
Other than installing the battery there are no installation or commissioning requirements.
TRAINING REQUIREMENTS
Where staff has experience with these types of devices, no user training will be required.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements. The device should be checked annually by technical staff to ensure its proper functioning.
COMPONENT OF A KIT
No
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
Self-declared CE under the EU MDR 2017/745 as Class I device.
SAFETY AND PRODUCT STANDARDS
- ISO 10942:2006 Ophthalmic instruments — Direct ophthalmoscopes. Preference is given to products compliant with this standard.
- ISO 15004-1 Ophthalmic instruments — Fundamental requirements and test methods — Part 1: General requirements applicable to all ophthalmic instruments.
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- EN ISO 15223-1 (EN 980) Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements.
NOMENCLATURE
GMDN Code: 46786
Related Products