S0845157
Otoscope, set.
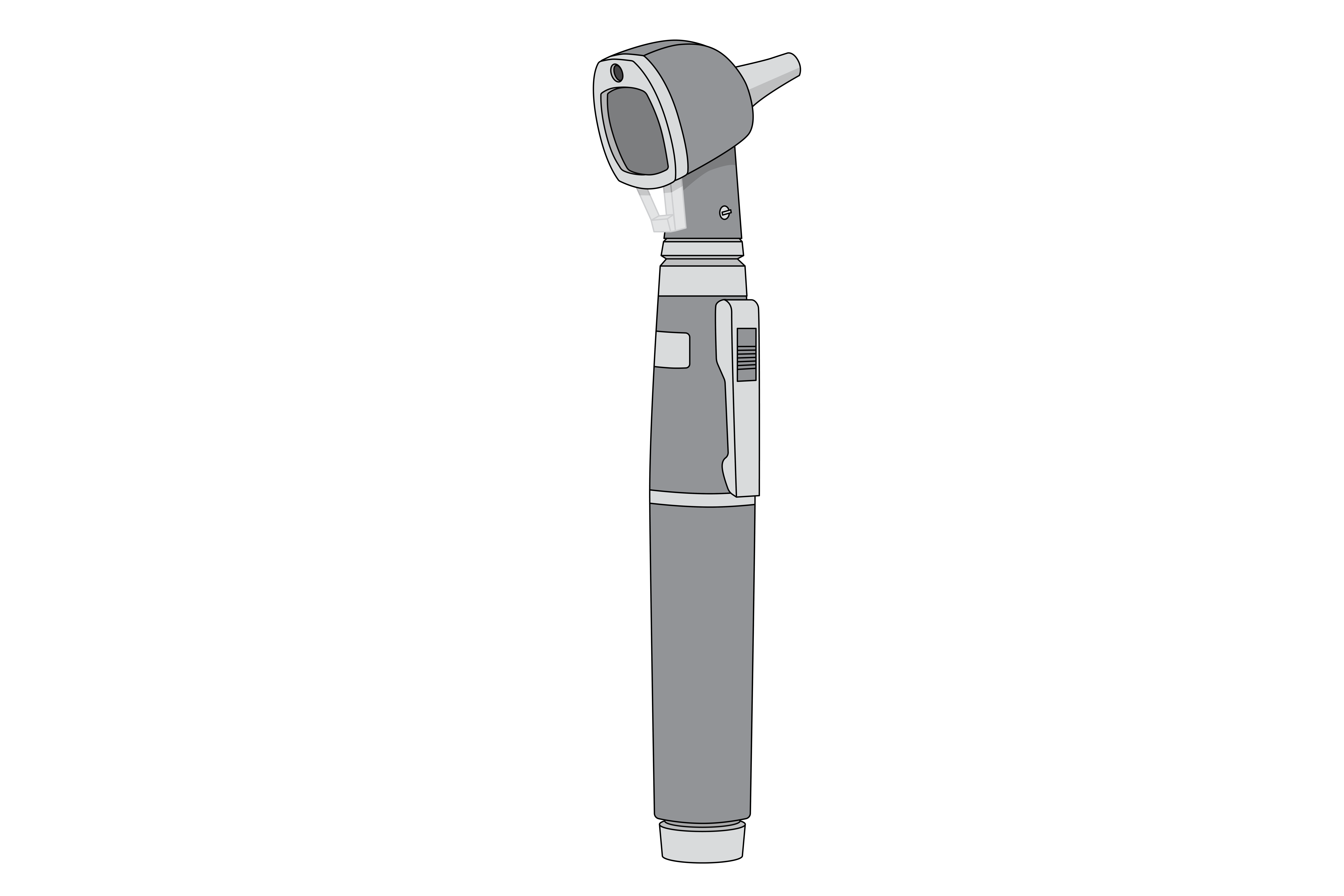
Otoscope set for visual examination of the eardrum and the outer ear, comes including two sets of four different sized reusable specula, a bulb and tube, a storage case, and required batteries.
Indicative Price 58.05 USD
GENERAL DESCRIPTION
Otoscope including all required accessories.
INTENDED USE
An electrically powered, hand-held device designed for examination of the outer ear canal and tympanic membrane (eardrum) by direct viewing through the ear opening. It consists of a main unit (commonly referred to as the head) with a built-in light source intended to illuminate the interior of the ear canal with a detachable cone-shaped tube (speculum) inserted in the ear canal; it may facilitate the application of air pressure for pneumatic otoscopy. A dedicated power supply handpiece and/or an insufflation bulb may be included with the head.
TECHNICAL SPECIFICATIONS
Handle with a built-in LED light source in a mounted head.
Including rotatable viewing lens with a magnification between 3 times.
Colour temperature: Cool white in the range 3,200-4,000K.
Including reusable, autoclavable specula.
Specula are cleanable with alcohol wipes
Handle contains on/off switch.
Includes a port to connect an insufflation bulb.
Uses 2 x type AA Alkaline batteries as a power source.
Is able to withstand frequent cleaning and disinfecting with hospital grade products.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x Plastic protective hard case containing full set.
2 x Sets of four reusable plastic specula different diameters ranging from 2 to 5mm.
1 x Set suitable disposable alkaline batteries.
1 x Bulb and tube for pneumatic tests.
ESTIMATED LIFE SPAN
Depending on the supplied model five to ten years.
WARRANTY
Two years.
ENVIRONMENTAL CONDITIONS
Storage conditions: -10 - 55°C / 10 - 95% RH.
Operating conditions: 10 - 35°C / 30 - 75% RH.
Atmospheric pressure: 700hPa – 1060hPa.
WEIGHT AND VOLUME (Packaged)
Weight: 529 gr.
Volume: 0.988 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
Other than installing the battery there are no installation or commissioning requirements.
TRAINING REQUIREMENTS
Where staff has experience with these types of devices, no user training will be required.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements.
COMPONENT OF A KIT
S9901029 - IEHK2017,kit,suppl.2-equipment.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
Self-declared CE under the EU MDR 2017/745 as Class I device.
SAFETY AND PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- EN ISO 15223-1 (EN 980) Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements.
NOMENCLATURE
GMDN Code: 12849
Related Products