S0845216
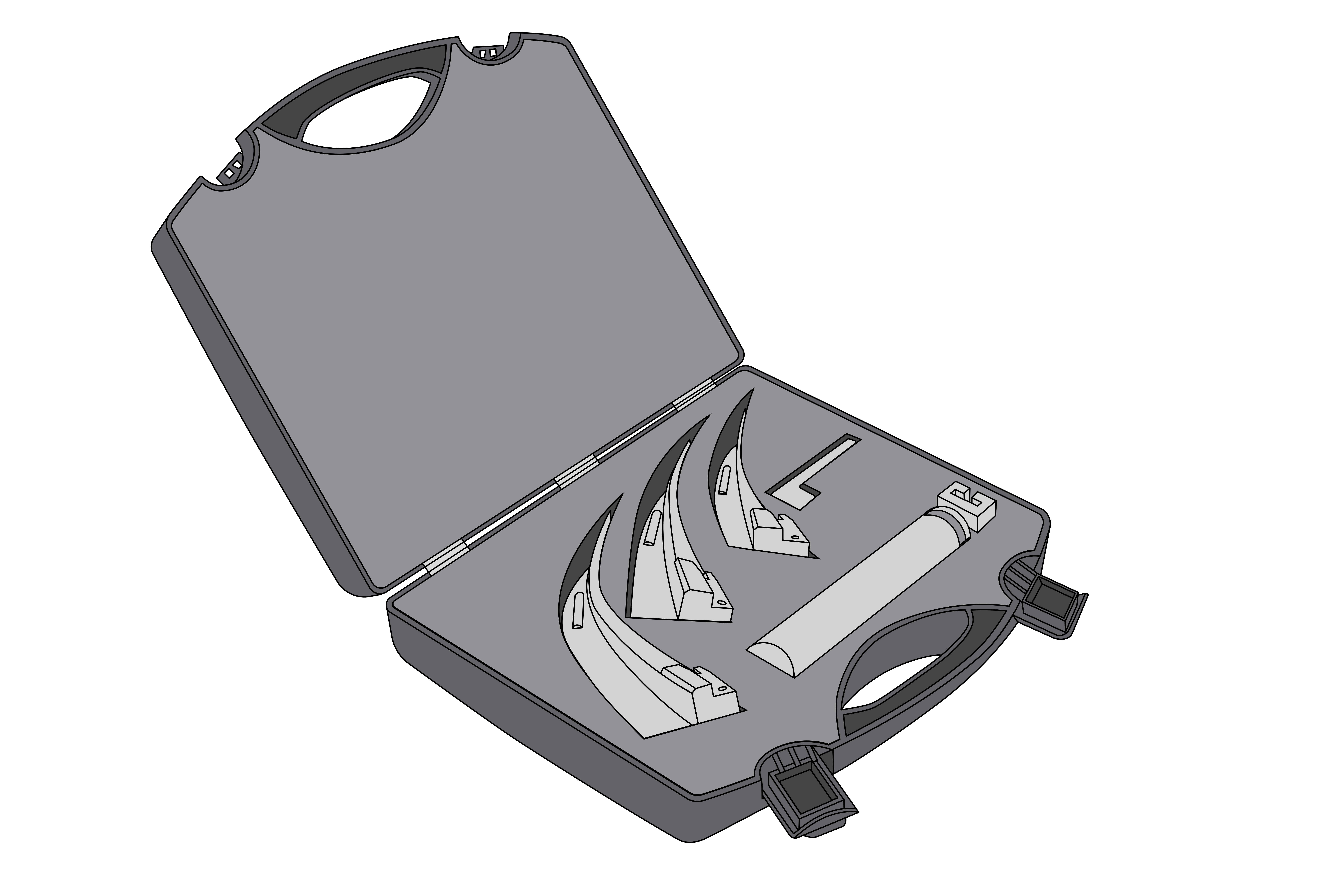
Laryngoscope,adult,child,set
Laryngoscope set for adults and paediatric patients, exists of a handle containg batteries and one straight, Miller type and three types curved MacIntosh depressor blades.
Indicative Price 145.80 USD
GENERAL DESCRIPTION
Laryngoscope set for adults and children. To manipulate the tongue and enable a clear view of the trachea for surgical/mechanical ventilation/intubation procedures.
INTENDED USE
A hand-held device intended to be used by medical professionals to manipulate the tongue, preventing it from obstructing the oropharynx and enabling a clear view of the trachea for the insertion of an endotracheal (ET) tube prior to the delivery of inhalation anaesthesia and/or ventilation. It has a handle containing batteries to power its light (a small built-in light bulb or fibreoptic light) for airway illumination, and a curved or straight blade of various designs and lengths that can be hinged/interchanged or integral.
TECHNICAL SPECIFICATIONS
Includes a cylindrical handle, slightly ribbed, single piece, Ø > 25 mm
The handle allows fitting of interchangeable blades of different sizes.
The handle contains a robust on/off switch.
Handle is made from non-ferrous material and sealed against ingress of liquids.
Equipped with LED technology light source.
Uses fibre optic for transmission of the light to the tip of the blade.
Stud contact, fitting various sizes and types of depressors, compliant with ISO 7376.
Blades are able to withstand autoclaving.
With a set of four blades made from stainless steel:
- MacIntosh type: Curved Nr 2, length 110mm ± 5 mm.
- MacIntosh type: Curved Nr 3, length 135mm ± 5 mm.
- MacIntosh type: Curved Nr 4, length 155mm ± 5 mm.
- Miller type: Straight Nr 1, length 100mm ± 5 mm
Designed for frequent and easy disassembly and disinfection with hospital-grade products.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x protective case with space for one handle and 2 blades.
2 x AA type alkaline batteries or, 2 x C type alkaline battery depending on the supplied brand.
ITEMS REQUIRED, BUT NOT SUPPLIED
S0383000, Tube,endotrach,3,w/o cuff,ster,disp.
S0383010, Tube,endotrach,3.5,w/o cuff,ster,disp.
S0383020, Tube,endotrach,6.5,w/cuff,ster,disp.
S0383030, Tube,endotrach,7,w/cuff,ster,disp.
S0383040, Tube,endotrach,7.5,w/cuff,ster,disp.
S0383050, Tube,endotrach,8,w/cuff,ster,disp.
ESTIMATED LIFE SPAN
Depending on the supplied brand three to five years.
WARRANTY
Two years.
ENVIRONMENTAL CONDITIONS
Storage conditions: 0 - 50°C / 85% RH.
Operating conditions: 10 - 40°C / 85% RH.
WEIGHT AND VOLUME (Packaged)
Weight: 920 gr.
Volume: 8.50 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
Other than installing the battery there are no installation or commissioning requirements.
TRAINING REQUIREMENTS
Where staff has experience with these types of devices, no user training will be required.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements.
RELATED PRODUCTS
S0845215, Laryngoscope,neonate,set
COMPONENT OF A KIT
No
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Self-declared CE under EU MDR 2017/745 as Class I device.
SAFETY AND PRODUCT STANDARDS
- ISO 7376:2020. Anaesthetic and respiratory equipment — Laryngoscopes for tracheal intubation.
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests
- IEC 60601-1-6:2010 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability
- EN ISO 15223-1 (EN 980) Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements.
NOMENCLATURE
GMDN Code: 15076
Related Products