S9901027
IEHK2017,kit,suppl.1a-medicines
Interagency Emergency Health Kit 2017 (IEHK2017) Supplementary module 1a
– controlled medicines
Indicative Price 282.52 USD
General Description
Interagency Emergency Health Kit 2017 Supplementary module 1a – controlled medicines
Technical specifications:
Kit contents/Description:
20 x S1543625 Diazepam inj 5mg/ml 2ml amp/BOX-10pt
1 x S1532305 Ketamine inj 50mg/ml 10ml vial/BOX-25nt
5 x S1555950 Morphine sulph.inj 10mg/ml 1ml/BOX-10nt
1 x S1569650 Naloxone inj 400mcg/ml 1ml amp/BOX-10
3 x S1543630 Diazepam 5mg tabs/PAC-100pt
4 x S1555951Morphine sulfate 10mg IR tabs/PAC-56 nt
Instruction for use
The IEHK2017 supplementary module 1a-controlled medicines is an integral part of the IEHK2017 Supplementary module 1 – medicines.
The IEHK2017 supplementary module 1a-controlled medicines contains medicines that normally
need import authorizations (narcotic/psychotropic substances). Since this kit is usually urgently required and needs to be shipped within 24 hours, it does not allow time to apply for import and export authorizations.
Therefore, until further notice, Supply Division will not require import authorizations for this kit. However, Country Offices ordering or receiving this kit are advised to investigate if their national authorities require an import authorisation. For these shipments, Supply Division will complete the "Notation Form for Emergency Supplies of Controlled Substances" and forward it to the DMA (Danish Medicines Agency). Please refer to Supply Manual, Chapter 4, Section 2- Annex 2 - Ordering of narcotic and/or psychotropic pharmaceutical products.
This kit must only be used by physicians and other professional health workers at the first referral level of health services.
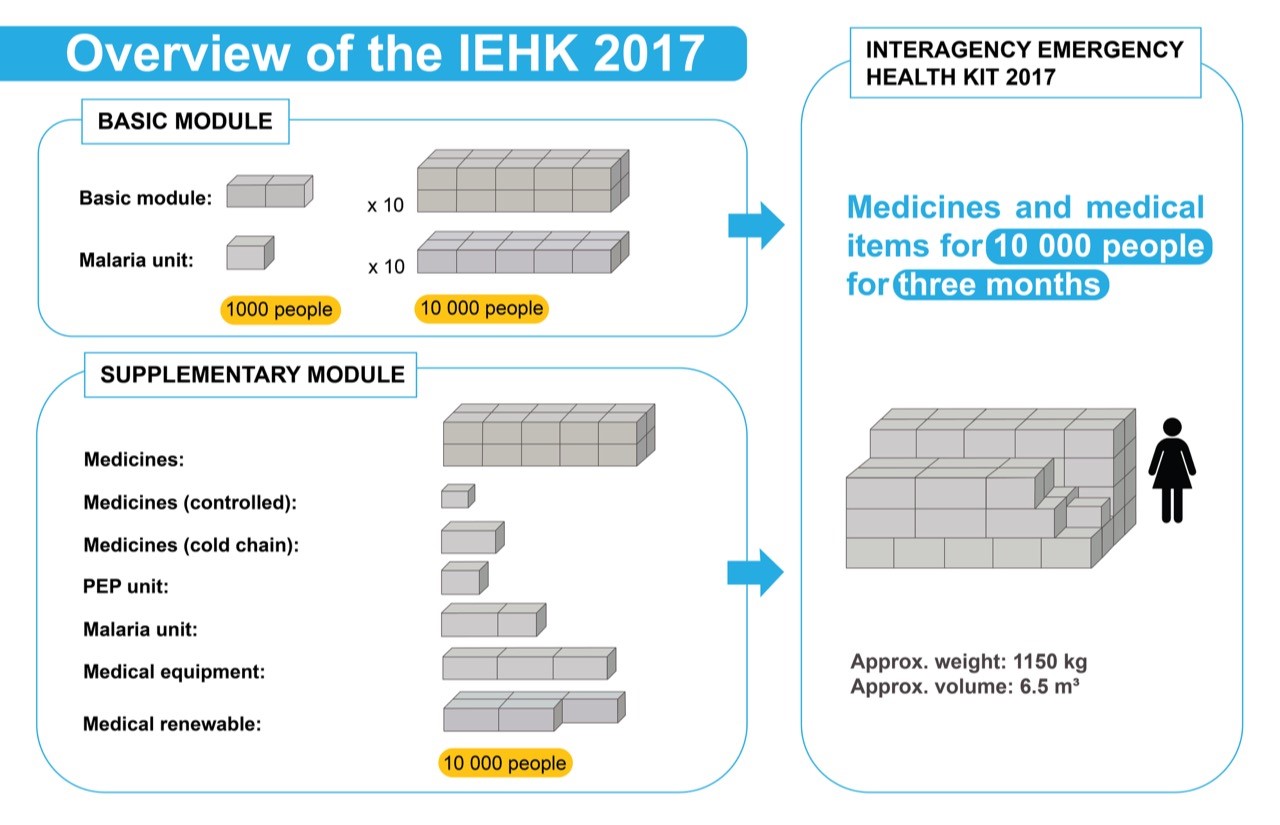
Note The IEHK2017 kit, supplementary module 1a – controlled medicines (as part of the IEHK2017 suppl.1 – medicines) is one part of the "Interagency Emergency Health Kit 2017-Complete".
The "Interagency Emergency Health Kit 2017-Complete" is designed to meet the initial primary health care needs of a population (10.000 persons for 3 months), or a displaced population without medical facilities, or a population with disrupted medical facilities in the immediate aftermath of a natural disaster or during emergency situations.
Important Customers should order the "Interagency Emergency Health Kit 2017-Complete" to fulfil the objectives. For full information on the purpose, concept, contents and how to order the complete kit, please refer to Technical Bulletin No.12 (Click on UNICEF web catalogue toolbar: TECHNICAL BULLETINS).
ADDITIONAL / EMERGENCY RELATED INFORMATION
Emergency scenarios
This item is part of UNICEF Emergency supply list for health; reference is made to "Assumed Scenario of complex emergency or natural disaster with little/no health services".
Transport and Storage
Do not store above 25°C
The kit contains controlled substances
Batch managed: yes
This kit is therefore only supplied via air shipment.
Guidelines for use:
WHO publication -The Interagency Emergency Health Kit 2017 with more information
A full list of technical guidelines for health emergencies
WHO Model Formulary 2008
MSF Essential Drugs
M SF Clinical Guidelines
Related Products