S0002017
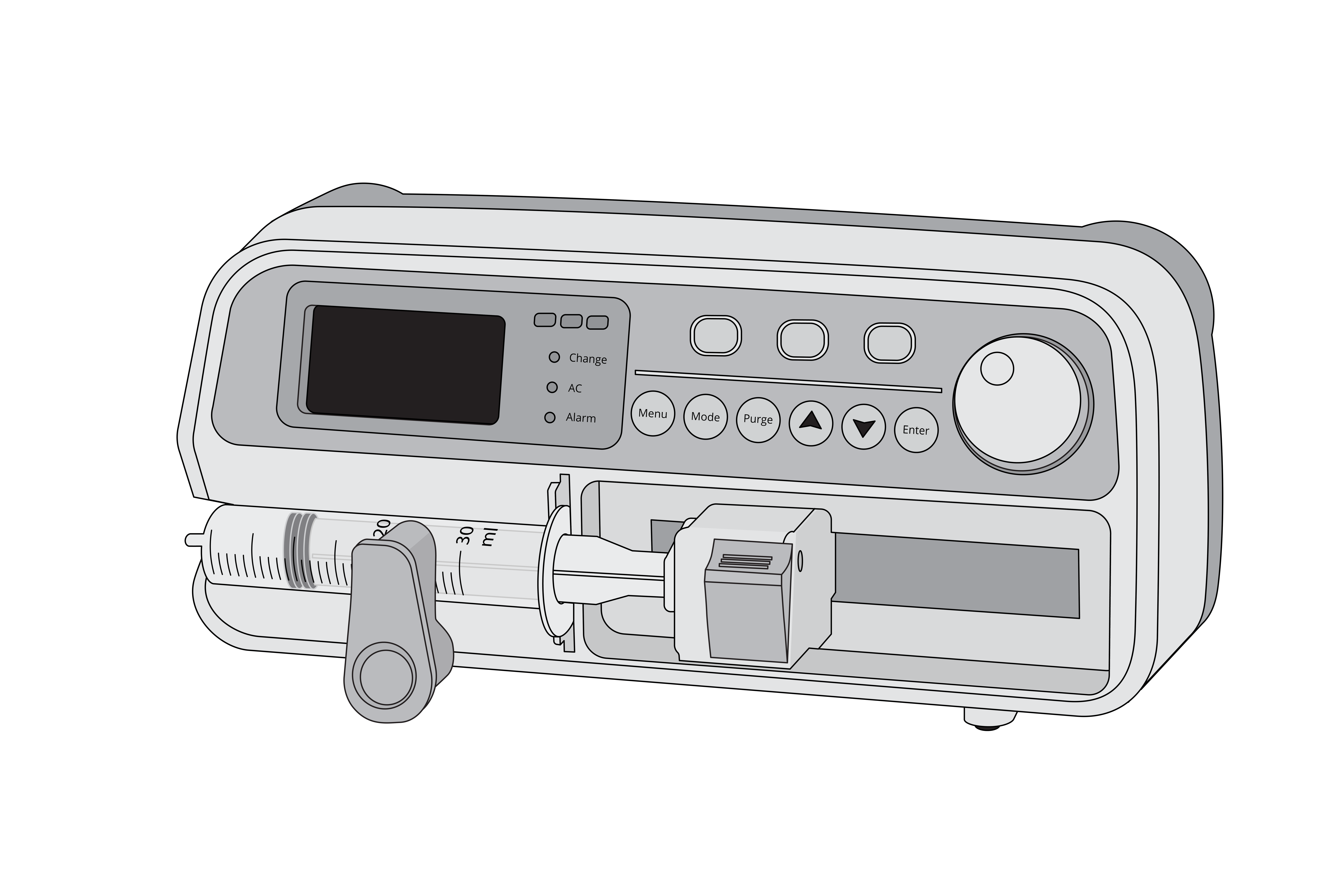
Syringe pump,with accessories
Syringe pump, single channel, AC and battery powered, with accessories
Indicative Price 424.20 USD
GENERAL DESCRIPTION
Volume controlled portable syringe pump for precise administration of fluids.
INTENDED USE
Syringe infusion pumps are used to administer intravenous (IV) fluids such as antibiotics, regional anaesthetics, antiarrhythmic medications, and chemotherapeutic agents. Syringe pumps ensure highly accurate volume delivery and consistent flow for small volumes (≤60 mL) of potent pharmacologic agents that are typically delivered at flow settings between 0.5 and 10 mL/hr.
TECHNICAL SPECIFICATIONS
Open system, compatible with all standard brands of syringes.
Compatible with 10, 20, 30 and 50mL capacity syringes at a minimum.
Continuous delivery, linear motor and plunger driven.
The unit is equipped with occlusion detection.
Self-test is performed each time the device is switched on.
User programmable for syringe size, infusion volume, time, and flow rate.
Automatic calculation of third parameter when user enters in other two (volume, time, and flow rate).
Minimum guaranteed flow rate of 0.1-1300mL/hr, depending on syringe size.
Minimum Keep Vein Open (KVO) rate of 0.1-1mL/hr but never greater than programmed flow rate.
Volume delivered with an accuracy of at least 3%.
Maximum pressure of at least 17.4 PSI / 120 kPa.
User adjustable high pressure/occlusion settings.
Display includes start/stop, volume limit, flow rate and volume so far delivered.
System reports with audio-visual alert on operational status such as, but not limited to ready, end-of-injection.
System reports with audio-visual alert on malfunctions such as, but not limited to syringe position, occlusion, low/high flow.
System reports with audio-visual alert on low battery status.
Ability to silence audio alarms for maximum of 2 minutes.
Built in battery with a capacity to run the unit for 7 hours at 5mL/hr flow rate.
Automatic switch from mains to battery in case of power failure.
Automatic battery charge when mains connection is re-established.
Capable of being mounted on mobile pole/(roll) stand, bed rail and wall-mounted rails.
Designed for frequent and easy dismount and disinfection with hospital-grade products.
Power requirements: 240 Volts - 50/60 Hz (110 Volts can be supplied if specifically requested).
SUPPLIED WITH
Instructions for assembly, use and maintenance in English.
1 x Spare battery pack.
1 x Mounting bracket for fixation to standard bed/wall rail and mobile pole/stand.
1 x Set of spare fuses, if applicable.
ITEMS REQUIRED, BUT NOT SUPPLIED
S0782405, Syringe,disp,5ml,ster/BOX-100.
S0782413, Syringe,disp,10ml,ster/BOX-100.
S0782420, Syringe,disp,20ml,ster/BOX-80.
ESTIMATED LIFE SPAN
5 years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 10°C - 40°C / 90% RH.
- Storage conditions: 0°C - 50°C / 90% RH.
- Atmospheric pressure: 860 - 1060 hPa.
WEIGHT AND VOLUME
Weight: 4.559 kg.
Volume: 35.000 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS
S0002034 Infusion pump,with accessories.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-2-24:2012 Medical electrical equipment - Part 2-24: Particular requirements for the basic safety and essential performance of infusion pumps and controllers.
- EN 62304:2006 Medical device software — Software life cycle processes.
NOMENCLATURE
- GMDN code: Syringe pump (13217).
- UMDNS code: Infusion Pumps, Multitherapy (13217).
Related Products