S0002025
Electrosurgical unit,w/access
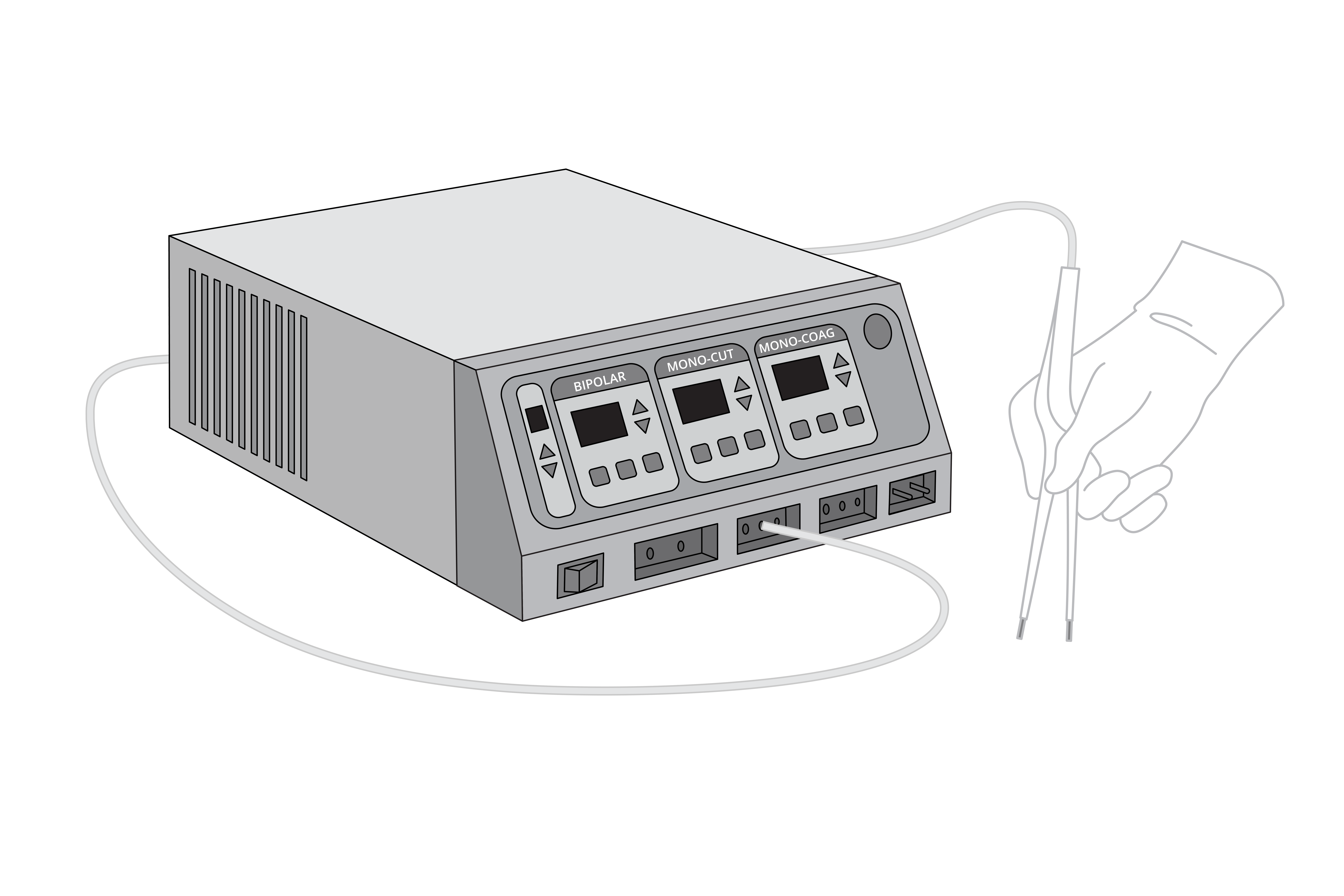
Electrosurgical unit, for general surgery, mono- and bi-polar, including a trolley and accessories.
Indicative Price 5,418.77 USD
GENERAL DESCRIPTION
Electrosurgical unit, for general surgery including a trolley.
INTENDED USE
Electrosurgical units are used for surgical cutting and for controlling bleeding by causing coagulation (haemostasis) at the surgical site. They deliver high-frequency electrical current through an active electrode tip, causing vaporization, desiccation, or charring by resistive heating in the target tissue. Electrosurgery is useful for nearly all surgical procedures, both open and laparoscopic. The haemostatic effect of electrosurgery makes it useful for procedures on organs with oozing capillary beds, such as the liver, spleen, thyroid, and lungs, as well as for open-heart procedures in which large amounts of anticoagulants are used.
TECHNICAL SPECIFICATIONS
Solid-state/microprocessor-controlled frequency generator.
Monopolar and bipolar outputs, electrically isolated from ground.
Minimum output frequency is higher than 400kHz.
Monopolar modes include pure cut, blend, and coagulate (soft, contact and spray).
Bipolar mode includes coagulate and cut mode.
Maximum monopolar cut power output maximum 300 W.
Maximum monopolar coagulation power output maximum 100 W.
Maximum bipolar power output maximum 100 W.
Hand switch mode when button-activated probes are connected.
Foot switch that can operate in monopolar and bipolar modes.
Yellow buttons/pedals for cut and blue buttons/pedals for coagulate.
Grounding pad/return electrode monitored for patient connection.
Front panel allows mode selection, power settings and on/off.
Display shows output power, system errors and electrode failure.
Automatic shut off/generator deactivation on grounding pad/return electrode connection failure.
Audible and visual indicators of activation and alarms.
Self-test mode.
Protection against defibrillator discharges.
Power requirements: 100 - 240 Volts - 50/60 Hz (indicate exact requirements at the time of ordering).
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x Plastic protective dustcover.
1 x Foot switch, two pedals, yellow and blue, with connecting cable.
2 x Grounding pad/return electrodes, reusable, with 3m connecting cable (adult & child).
2 x Monopolar electrode handle, reusable, foot switch controlled, with connecting cable.
2 x Monopolar electrode handle, reusable, finger switch controlled, with connecting cable.
1 x Set different monopolar reusable electrodes (needle, blade, ball, and loop).
2 x Bipolar forceps, reusable, foot switch controlled, with connecting cable (short, straight, tip-angled).
2 x Bipolar forceps, reusable, foot switch controlled, with connecting cable (long, straight, tip-angled).
1 x Trolley on 4 antistatic swivel castors, 2 with brakes, fit with a drawer and storage for foot pedal/switch.
ESTIMATED LIFE SPAN
5 years.
WARRANTY
Depending on the model one or two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 10°C - 40°C / 30% – 75% RH.
- Storage conditions: -10°C - 60°C / 30% – 90% RH.
- Atmospheric pressure: 700 ~ 7060 hPa.
WEIGHT AND VOLUME
Weight: 28 kg.
Volume: 160.00 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-1-6:2010 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability.
- IEC 60601-2-2 Medical Electrical Equipment – Part 2-2: Particular requirements for the safety of high frequency surgical equipment.
NOMENCLATURE
- GMDN code: General-purpose electrosurgical diathermy system (44776).
- UMDNS code: Electrosurgical Units, Monopolar/Bipolar (18231).
Related Products