S0002031
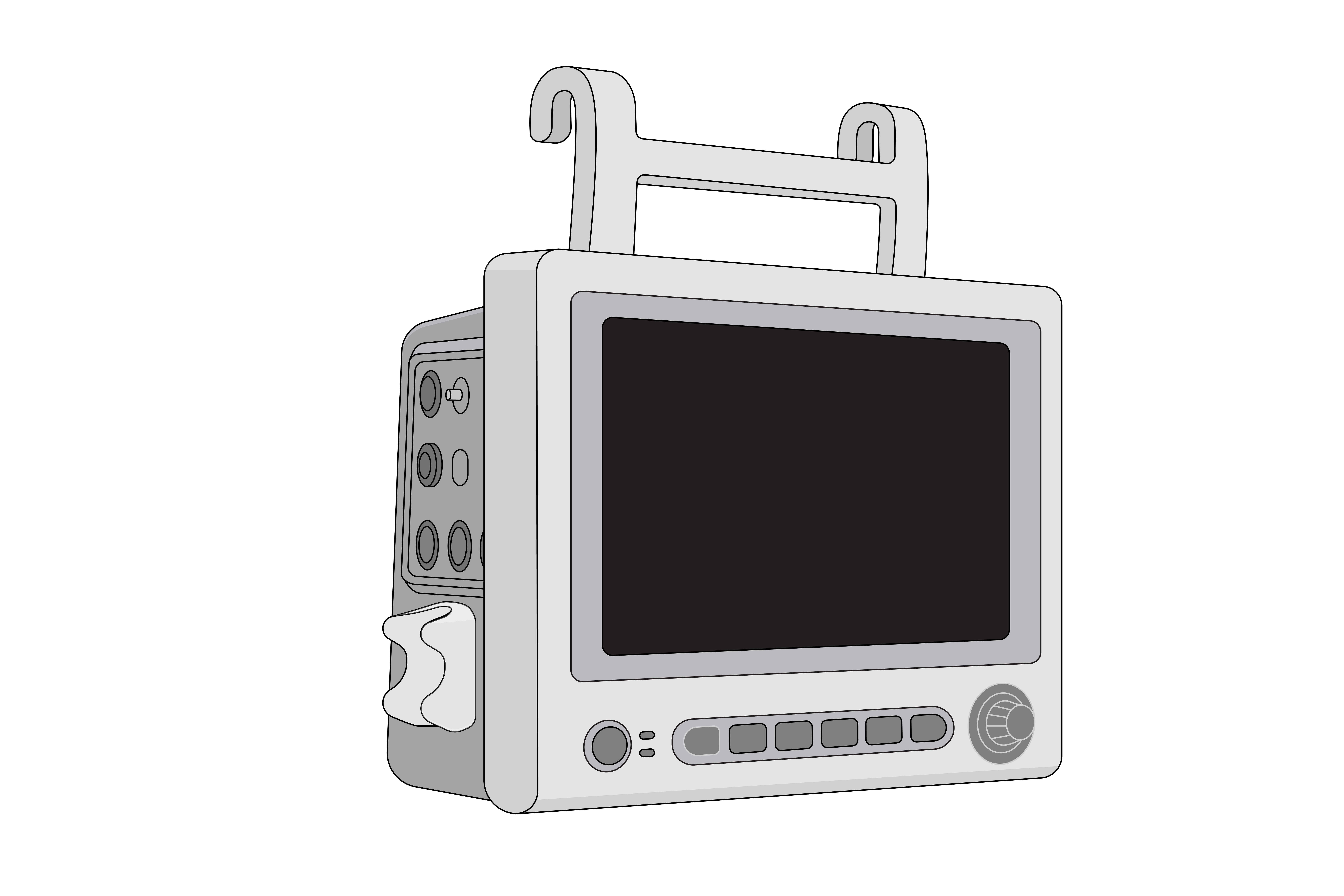
Monitor,patient,portable,w/access
Patient vital signs monitor, 6 physiological parameters; ECG, heart rate, respiration rate, Non-invasive blood pressure and pulseoximetry and temperatur. Suitable for adult, paediatric and neonatal patients, AC and battery powered, including accessories.
Indicative Price 1,615.40 USD
GENERAL DESCRIPTION
Patient vital signs monitor, 6 parameters, suitable for adults, paediatric/infants and newborn patients.
INTENDED USE
Vital signs monitors are used to measure basic physiologic parameters and track the status of low-acuity patients (patients found in medical-surgical areas, emergency departments, and ambulatory care centres) so that clinicians can be informed of changes in a patient's condition.
TECHNICAL SPECIFICATIONS
Monitoring 6-parameters: ECG and Heart Rate (HR), Respiratory Rate (RR), Sp02, non-invasive blood pressure, and Temperature.
Adjustable signal amplitude and sensitivity.
Colour flat panel display ≥ 10.4 inches.
Multi-waveform and parameters visualization, up to 7 wave forms simultaneously.
Ability to remove unwanted parameters from display.
Defibrillator sync and protection.
Patient information and trend internal database.
Ability to save a minimum of 72 events.
Trend storage ≥ 24 hours.
Data and network interface, LAN, USB or equivalent.
Suitable for standard bed/wall rail and pole stand mount.
Robust design for use in demanding environment.
Designed for frequent and easy dismount and disinfection with hospital-grade products.
Automatic self-test and continuous system monitoring.
Built-in rechargeable battery, autonomy of at least 2 hours.
Automatic switch to battery in case of power failure, automatic recharge on connection to mains.
ain cable at least 3 meter long.
Electrical protection provided by fuses in both live and neutral supply lines.
Power requirements: 100 - 240 Volts - 50/60 Hz (not necessarily in a single unit).
ELECTROCARDIOGRAM (ECG) AND HEART RATE (HR)
The ECG is derived from a minimum of 5-leads I, II, III, aVR, aVL, aVF, V.
The unit allows for a simultaneously display of a minimum of 2 ECG traces.
The accuracy of the HR is equal or better than ± 1 bpm or 1%.
The resolution of the HR is equal or better than: 1 bpm.
The unit provides for S-T analyses on ECGs.
The unit provide for arrhythmia analysis on the ECGs.
The unit is equipped with a standardising marker at 1 mV.
The adult HR measurement range is equal to or better than: 15 – 300 bpm.
The paediatrics/neonates HR measurement range is equal to or better than: 30 – 300 bpm.
RESPIRATION RATE (RR)
The adult RR measurement range is equal to or better than: 6 – 120 bpm.
The paediatric/neonatal RR measurement range is equal to or better than: 6 – 150 bpm.
68.1 Select your answer from the below drop-down menu.
68.2 Indicate here the actual specification of the offered unit.
The resolution of the RR is equal to or better then: 1 bpm.
NON-INVASIVE BLOOD PRESSURE (NIBP)
The NIBP is measured through the oscillometric step deflation method.
The unit supports manual and automatic modes.
The unit allows for adjustable inflation pressure.
The NIBP adult range in minimum diastolic and maximum systolic values should be equal or better than: 10 – 270 mmHg.
The NIBP paediatric NIBP measurement range in minimum diastolic and maximum systolic values should be equal or better than: 10 – 200 mmHg.
The BP neonatal measurement range in minimum diastolic and maximum systolic values should be equal or better than: 10 – 135 mmHg.
The resolution of the NIBP is equal to or better than: 1 mmHg or better.
The minimum average error for the NIBP is: ±5 mmHg (0.7 kPa), Standard deviation: ≥ 8 mmHg (1.1 kPa).
OXYGEN SATURATION (SPO₂)
The SpO₂ measurement range is equal to or better than: 1 – 100%.
The resolution of the SpO₂ is equal to or better than: 1% or better.
The accuracy of the SpO₂ is equal to or better than: ± 3% between 70% - 100%.
The HR detection range derived from the SpO₂ is equal to or better than: 25 – 250 bpm.
The accuracy of the HR detection is equal to or better than: ± 2 bpm or ± 2%, whichever is greater (static).
TEMPERATURE (T)
The temperature measurement range is equal to or better than: 0 - 50°C.
The temperature resolution is equal to or better than: 0.1°C or better.
The temperature accuracy is equal to or better than: ± 0.1°C.
ALARM FUNCTIONIONALITIES
The unit provide audio-visual alarms for all monitored parameters
The unit allows for user pre-set of high and low alarms for all monitored parameters for each different patient category.
The alarm has override and temporary silence functions.
The unit generates alarms for leads-off or sensor disconnect or sensor failure.
The unit is provided with an apnoea alarm.
The unit is equipped with an alarm for AC status and low battery.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English.
1 x Plastic protective dustcover.
1 x wall mount bracket.
1 x spare rechargeable battery pack.
1 x set of spare fuses, if required.
NIBP accessories:
- 3 x NIBP hoses (1 x neonatal, 1 x paediatric, 1 x adult).
- 3 x blood pressure cuffs (1 x neonatal, 1 x paediatric, 1 x adult).
ECG accessories:
- 2 x sets of patient cable terminals (1 x neonatal/paediatric, 1 x adult).
- 2 x sets of electrodes (1 x neonatal/paediatric, 1 x adult).
Temperature sensors:
- 2 x skin temperature probes including cable.
SpO₂ transducers, including connection cable:
- 2 x adult size, reusable clip-on type.
- 2 x paediatric size, reusable clip-on type.
- 3 x neonatal size, reusable clip-on type.
- 10 x neonatal size, single-use, wrap-around type.
OPTIONAL ACCESSORIES AVAILABLE
At extra costs the below optional feature can be requested:
- Two channels for invasive blood pressure monitoring.
ESTIMATED LIFE SPAN
5 – 7 years depending on the model.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 10°C - 40°C / 15% – 85% RH.
- Storage conditions: -20°C - 55°C / 15% – 90% RH.
- Atmospheric pressure: 700 ~ 1060 hPa.
WEIGHT AND VOLUME
Weight: 10.7 kg (without battery).
Volume: 92.63 dm³
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-1-6:2010 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability.
- IEC 60601-1-9:2007 Medical electrical equipment - Part 1-9: General requirements for basic safety and essential performance - Collateral Standard: Requirements for environmentally conscious design.
- IEC 60601-1-8 :2012 Part 1-8: General requirements for basic safety and essential performance - Collateral Standard: General requirements, tests, and guidance for alarm systems in medical electrical equipment and medical electrical systems.
- IEC 60601-2-27:2011 Part 2-27: Particular requirements for the basic safety and essential performance of electrocardiographic monitoring equipment.
- IEC 60601-2-34:2011 Part 2-34: Particular requirements for the basic safety and essential performance of invasive blood pressure monitoring equipment.
- IEC 80601-2-49:2018 Medical electrical equipment - Part 2-49: Particular requirements for the basic safety and essential performance of multifunction patient monitors.
NOMENCLATURE
GMDN©:
code:
- General-purpose multi-parameter bedside monitor (33586)
- Neonatal multi-parameter bedside monitor (35569)
Related Products