S0002032
Photo therapy unit,w/access
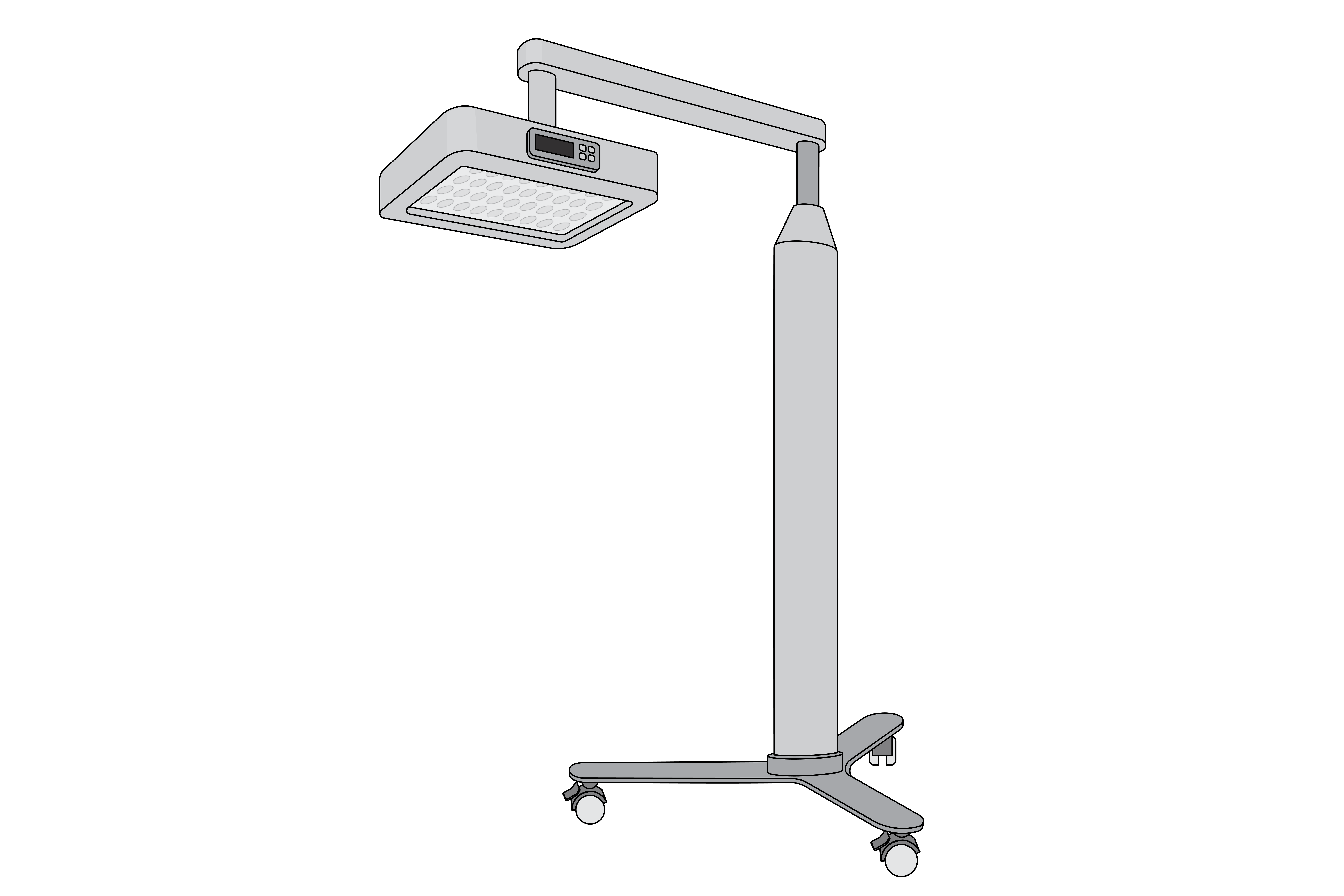
Phototherapy unit, LED, overhead, mobile, for the treatment of neonatal hyper-bilirubinaemia, AC powered, with accessories.
Indicative Price 2,247.59 USD
GENERAL DESCRIPTION
Mobile LED phototherapy unit AC powered including accessories.
INTENDED USE
Phototherapy units are used to treat hyperbilirubinemia, a condition characterized by high bilirubin concentrations in the blood. Bilirubin, a product of haemoglobin breakdown, remains in the body until the liver can convert it to a form that can be excreted. Jaundice, a yellowish discoloration of the skin, eyes, and mucous membranes, is a major symptom observed when bilirubin levels in blood rise above 5 mg/dL.
TECHNICAL SPECIFICATIONS
Sturdy and stable wheeled base with a low profile.
The wheeled base is provided with a minimum of three (3) or more anti-static swivel castors.
The base is provided with a minimum of two (2) or more brakes castors.
The unit has an adjustable height with a minimum range: 1.20 – 1.60 m.
The unit includes an arm to allow positioning (swivel) the unit above an incubator or a cradle.
The single head allows tilting up to a minimum of 45°.
All surfaces to be made of corrosion resistant materials resistant to hospital grade disinfection chemicals.
The unit should be equipped with an integrated, operator resettable, therapy timer.
The therapy timer indicates the elapsed time.
The therapy time is unaffected by power outages up to 10 minutes.
The unit includes a provision to safeguard patients from excessive temperatures utilizing a skin temperature sensor and alarms or cutting of the mains supply when limits are exceeded.
External surfaces of the unit shall never exceed 40 degrees either through an active cooling provision or through design of the unit.
Equipped with examination LEDs providing white light.
Power requirements: 100- 240 Volts – 50/60 Hz.
LIGHTING SYSTEM FEATURES:
Achieves the phototherapy for a single surface utilising LEDs only.
Blub lifetime at least 50,000 hrs.
The wavelength bandwidth should cover at a minimum 450 – 470 nm.
Minimum effective surface area ≥ 1,220 cm².
Standard phototherapy irradiance within range: 8 - 15 µW/cm²/nm.
Intensive phototherapy minimum irradiance range: ≥ 30 µW/cm²/nm.
Ultraviolet levels shall not exceed a maximum effective radiance of 0.1 μW/cm² for ultraviolet B radiation (280 to 315 nm).
Ultraviolet levels shall not exceed a maximum irradiance of 1,000 μW/cm² for ultraviolet A radiation (315 to 400 nm).
Near-infrared (780 to 1,400 nm) radiation shall not exceed safe limits.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x Plastic protective dustcover.
2 x replacement sets of fuses, if replaceable types are used.
10 x eyes protection masks newborn very small size.
10 x eyes protection masks neonatal small size.
10 x eyes protection masks neonatal medium size.
ESTIMATED LIFE SPAN
10 years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 10°C - 40°C / 30% – 90% RH.
- Storage conditions: -20°C - 60°C / 5% – 95% RH.
- Atmospheric pressure: 700 ~ 1060 hPa.
WEIGHT AND VOLUME
Weight: 25 kg
Volume: 233.00 dm³
ESTIMATED DELIVERY LEAD TIME
120 days
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS
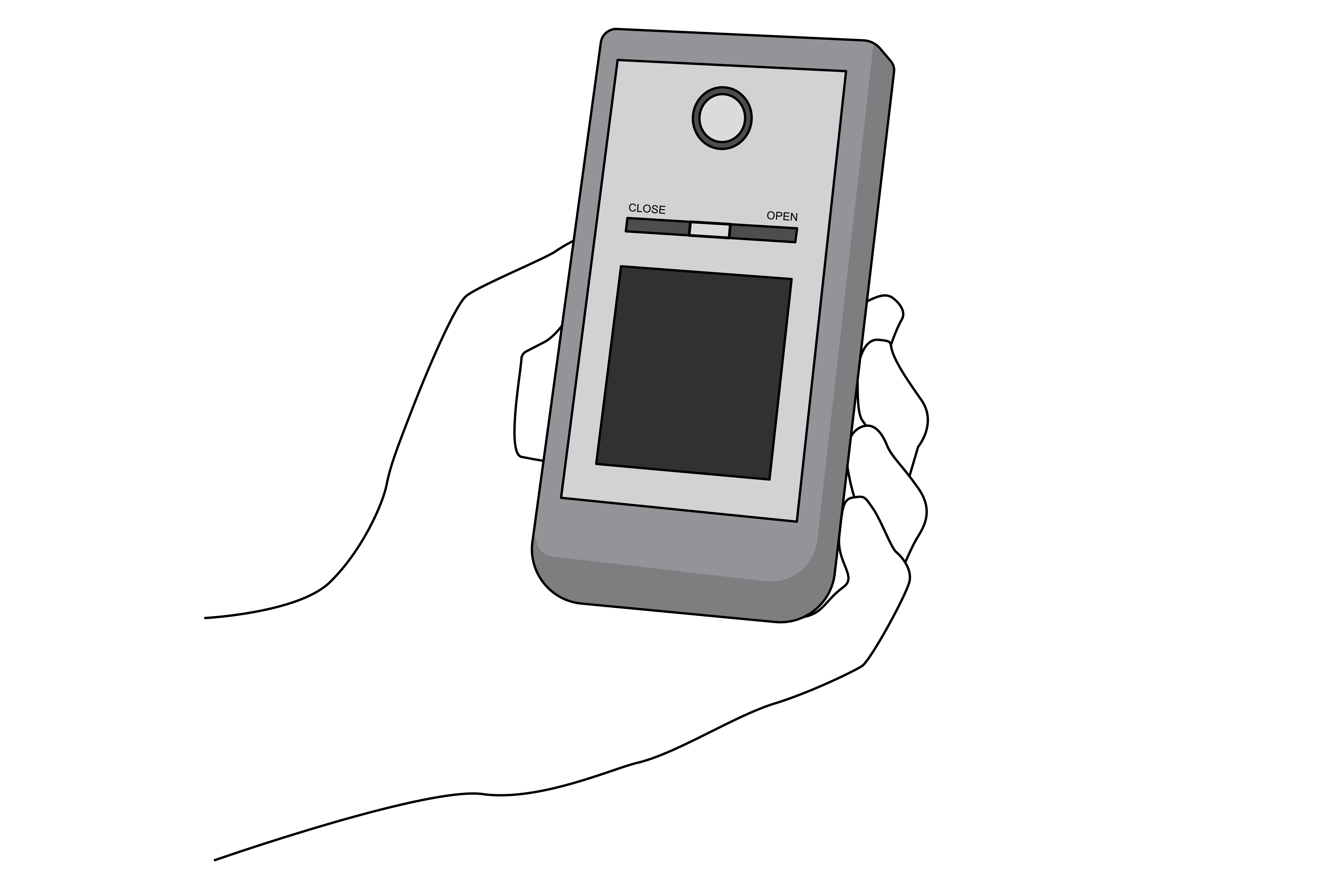
S0002018, Phototherapy irradiance meter.
S0845180, Protector, eye, neonatal phototherapy.
S0845181, Protector, gonad, neonatal phototherapy.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-1-6:2010 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability.
- IEC 60601-2-50 Medical electrical equipment - Part 2-50: Particular requirements for the basic safety and essential performance of infant phototherapy equipment.
NOMENCLATURE
- GMDN code: Overhead infant phototherapy unit (35239).
- UMDNS code: Phototherapy Units, Visible Light, Hyperbilirubinemia (17515).
Related Products