S0002061
Doppler,FHR detector,w/access
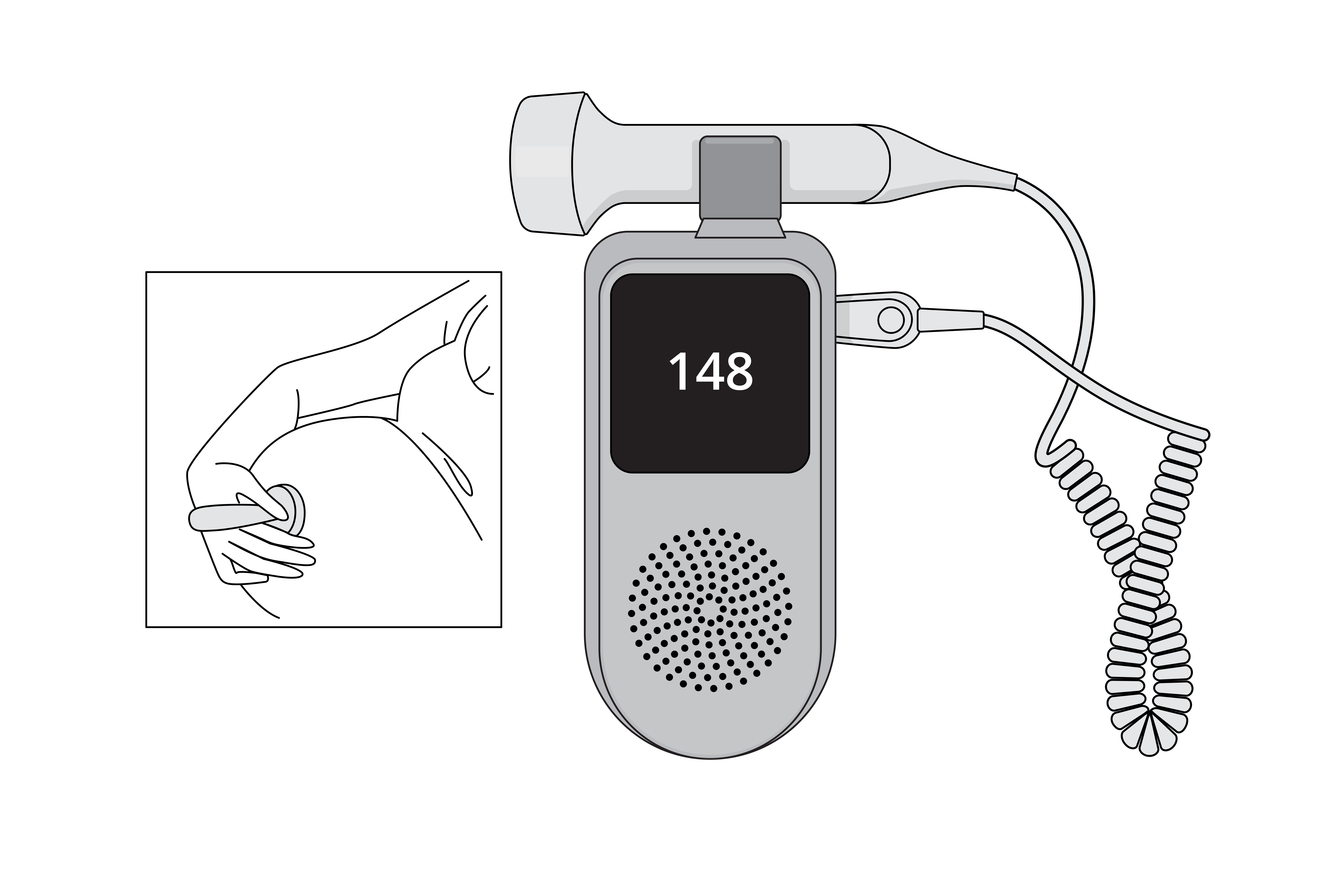
Foetal Heart Rate (FHR) doppler detector, with speaker, handheld, battery powered, with accessories.
Indicative Price 165.00 USD
GENERAL DESCRIPTION
Handheld doppler based foetal heart rate detector with amplifier loudspeaker.
INTENDED USE
Ultrasonic fetal heart detectors are low-cost devices used in a variety of healthcare settings to provide audible and visual information about the fetus. Certain conditions, such as vaginal bleeding or lack of fetal movement, can cause anxiety in the mother, making her question the health of her unborn baby. The primary purpose of the fetal heart detector is to provide quick reassurance of fetal well-being to both the mother and the healthcare worker. The fetal heartbeat cannot be heard with an obstetric stethoscope until 24 weeks after conception. Ultrasonic fetal heart detectors can easily detect fetal heart motion throughout the pregnancy, starting as early as 8 weeks.
TECHNICAL SPECIFICATIONS
Capable of detecting foetal heart rates (FHRs) in the range of 50 - 210 bpm, with 1 bpm resolution and 2 bpm accuracy.
Probe (transducer) with 2 MHz frequency with a probe attached via a cable.
The cable can be extended to a 25 cm length when stretched.
Probe detector head diameter at least 39 mm.
Handheld, weights less than 0.45 kg with probe and batteries.
Built-in speaker with volume adjustment.
LCD screen displays FHR, pulse indicator and battery status
Visual alerts for status, system error and low battery
Operates on 3 x AA batteries.
Battery life is suffiecient for 10 hours continuous use.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
2 x Tubes of ultrasound gel, approximately 350ml each. (These come with a lmited shelf life)
2 x Set of Alkeline LR6 batteries (separately packed).
1 x Soft, easy to clean carrying bag.
OPTIONAL ACCESSORIES AVAILABLE
At extra costs additional optional features can be requested:
- The probe is interchangeable and probes with other frequencies are available: 4Mhz, 5Mhz and 8Mhz.
ESTIMATED LIFE SPAN
Seven years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 5°C - 40°C / 25% – 80% RH.
- Storage conditions: -20°C - 55°C / 25% – 93% RH.
- Atmospheric pressure: 860 ~ 1060 hPa.
- Ingress protection rating: Probe only IPX8.
WEIGHT AND VOLUME (packaged)
Weight: 1.60 kg.
Volume: 8.00 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
There is no assembly or commisioning required for this device, other than instaling the batteries.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIa device
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-1-6:2010 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability.
- IEC 60601-2-37:2007+AMD1:2015 CSV Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment.
- IEC 61157:2007+AMD1:2013 CSV Standard means for the reporting of the acoustic output of medical diagnostic ultrasonic equipment.
- IEC 61266:1994 Ultrasonics - Hand-held probe Doppler foetal heartbeat detectors - Performance requirements and methods of measurement and reporting.
NOMENCLATURE
- GMDN code: Foetal heart detector, ultrasonic (35068).
- UMDNS code: Detectors, Fetal Heart, Ultrasonic (11696).
Related Products