S0782208
Safety box f.used syrgs/ndls 5lt/BOX-25
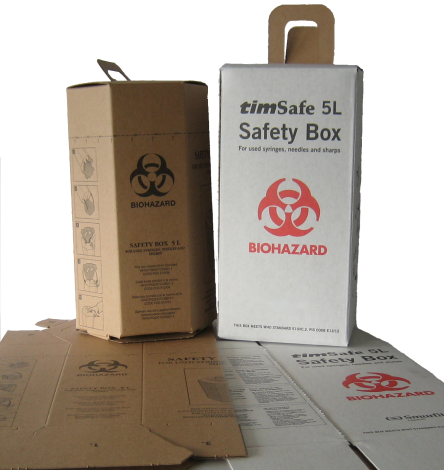
Safety Box for used Syringes/Needles, 5 liter, box of 25.
Indicative Price 22.50 USD
General Description:
Sharps safety boxes, constructed of cardboard or other materials, flat-packed, one piece with aperture and handle.
Intended use: safely and efficiently contain, transport and store used sharps injection devices until final destruction, safe disposal or recycling.
Technical specifications:
Minimum dimensions: The minimum height from the bottom of the container to the fill line is not less than 230mm.
Functionality: The safety box must safely contain contaminated sharps at the point of use; during temporary storage and during handling and transport to the point of treatment and final disposal.
Nominal Capacity: Boxes must accept no less than 20 units of 0.5ml AD syringes per nominal liter. Total storage capacity not less than 100 units/ 5L box. No syringe must protrude from the container or above the fill line and the box must be capable of being correctly and permanently closed without any risk of needle-stick injury
Maximum capacity: allowed to exceed the nominal capacity of 20 syringes per nominal liter provided no syringe must protrude from the container or above the fill line and the box must be capable of being correctly and permanently closed without any risk of needle-stick injury
Sharps aperture: Boxes fitted with a sharps aperture of 38 mm diameter, or 38mm width and breadth and placed at least 50 mm above the maximum recommended fill line marked on the exterior of the box. It is possible to close and seal the aperture at any time between empty and full to maximum capacity.
Handles: positioned above the fill line, does not obstruct access to the sharps aperture, sufficiently robust. Filled safety box is safely carried with one hand
During transport to the disposal site.
Color: color of unbleached sulphate board, or non-chlorine bleached white, or yellow.
Fill line: clearly marked on all vertical faces of the box, in black or red.
Shipping and storage volume before use:
Boxes must be supplied flat-packed or nested to minimize shipping and storage
volume.
Marking instructions on each safety box:
Bio-hazard marking: marked with the international bio-hazard warning not less
than 50mm diameter, printed in black or red on each of the front and back faces of
the box.
Pictorial instructions: without writing printed on two sides of the container showing:
• How to assemble the box.
• How to use the box as a container for contaminated sharps;
• Syringe disposal direction (needle down).
• How to close the sharps aperture when the box is full.
Standards:
Product and packaging: Product listed under WHO Performance Specification (PQS) Category E010 waste management equipment for immunization. Verified in accordance with PQS Verification Protocol E10/SB01-VP
Quality management system (QMS): Products and manufacturer must be certified and comply with ISO 9001: 2008 requirements (or equivalent).
Packaging and labelling
Primary packaging
None. One box not packed for construction at the field level.
Secondary packaging
25 boxes in one carton are flat-packed for ease of shipment and storage. Recyclable cardboard is to be used.
The packing is of a sturdy export quality, and of a commercial standard that will provide adequate protection of the goods for carriage by air, sea and/or road to final destinations worldwide, including remote locations under adverse climatic and storage conditions, and high humidity - i.e. not less than 17kN edge crush resistance with minimum 60% remaining with 90% humidity at a temperature of 40C (tropical conditions)
Labelling on the secondary packaging
Name and/or trade mark and address of the manufacturer.
Manufacturer's product reference.
Type of product and main characteristics
The number of units per secondary packaging
Purchase Order Number (optional for inner boxes);
UNICEF Material Number (If applicable);
Description of contents;
Quantity per carton;
Gross Weight;
Cubic Measurement;
Batch Number Reference (if applicable);
Manufacturing Date (if applicable);
Manufacturer's instruction for use, including construction instructions of the box.
Instructions for use shall be given either on the package or on a separate insert
Related products
S0002016Syringe,A-D,0.5ml,w/ndl/BOX-100
S0002013Syringe,A-D,BCG,0.05ml,w/ndl/BOX-100
S0002012Syringe,A-D,BCG,0.1ml,w/ndl/BOX-100
S0782311Syringe,RUP 2ml w/o ndl
S0782312Syringe,RUP 5ml w/o ndl
S0782313Syringe,RUP 10ml w/o ndl
S0782316Syringe,RUP,2ml,w/ndl,bi-pack/BOX-100
S0782317Syringe,RUP,5ml,w/ndl,bi-pack/BOX-100
S0782318Syringe,RUP,10ml,w/ndl,bi-pack/BOX-100
S0782319Syringe,RUP,SIP,1ml,w/ndl,25Gx1/BOX-100
S0782320Syringe,RUP,SIP,5ml,w/ndl/BOX-100
S0782321Syringe,RUP,2ml,w/ndl,fixed,21G/BOX-100
S0782322Syringe,RUP,5ml,w/ndl,fixed,21G/BOX-100
S0782323Syringe,RUP,10ml,w/ndl,fixed,21G/BOX-100
S0747420Needle,disp,19G,ster/BOX-100
S0747432Needle,disp,21G,ster/BOX-100
S0747440Needle,disp,22G,ster/BOX-100
S0747452Needle,disp,23G,ster/BOX-100
S0747445Needle,disp,25G,ster/BOX-100
S0782110Syringe,disp,2ml,w/ndl,21G/BOX-100
S0782111Syringe,disp,5ml,w/ndl,21G/BOX-100
S0782112Syringe,disp,10ml,w/ndl,21G/BOX-100
S0782203Syringe,disp,1ml,ster/BOX-100
S0782205Syringe,disp,2ml,ster/BOX-100
S0782405Syringe,disp,5ml,ster/BOX-100
S0782413Syringe,disp,10ml,ster/BOX-100
S0782425Syringe,disp,20ml,ster/BOX-100
S0002600Incinerator,medical infect. waste&access
-
Useful links
WHO, waste management equipment for immunization
Related Products