S0782111
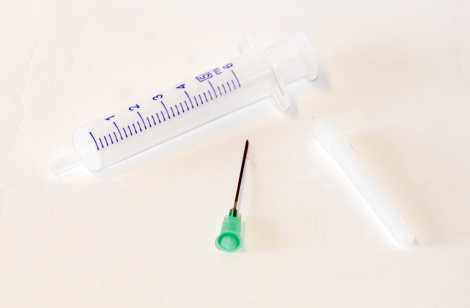
Syringe,disp,5ml,w/ndl,21G/BOX-100
Syringe, 5ml, sterile, with detached (bi-packed / mounted) needle, 21Gx1 1/2” (0.80 x 40mm), disposable, box of 100.
Indicative Price 3.26 USD
General Description:
Syringe, 5ml, sterile, with bi-packed needle, 21Gx1 1/2” (0.80 x 40mm), disposable, box of 100
Intended use: inject fluids into, or withdraw fluids from, the body
Technical specifications:
Syringe Components: Syringe, two pieces: barrel with Luer nozzle and piston/plunger or three pieces: barrel with luer nozzle, stopper and piston/plunger.
Syringe capacity: 5ml
Syringe graduation: Graduated scale on the barrel, easy to read. Scale intervals of 0.2 or 0.5ml. Graduation lines of 1.0ml volume increment conform to ISO 7886-1:2017.
Syringe Barrel: sufficiently transparent to allow easy measurement of the volume contained in the syringe and detection of air bubbles. Length with a maximum usable capacity of at least 10% more than the nominal capacity conform to ISO 7886-1:2017.
Barrel Nozzle: Luer lock or luer slip in accordance with ISO 80369-7:2021. The Luer nozzle shall be situated centrally.
Syringe Material: medical grade plastic (Polyethylene (PE)/ polypropylene (PP)/ polystyrene (PS).
Needle Components: Needle with base (Luer type fitting and complies with ISO 80369-7:2021), and protective cap.
Needle Material: Stainless steel conform to ISO 9626:2016.
Base and protective cap: medical grade plastic.
Measurements:External diameter expressed in Gauge and millimeters.
Length expressed in inch and millimeters.
Size selected: 21Gx1 1/2” (0.8x40mm).
Colour code: visible at the base of the needle and complies with ISO 6009:2016.
Syringe with needle: Syringe with bi-packed needle: Disposable.
Sterile, (ethylene oxide sterilization and compiles to ISO 11135:2019)
Standards:
Product and packaging conform to ISO 7886-1:2017, ISO 7864:2016, ISO 9626:2016, ISO 11135:2019.
Quality management system (QMS): Products and manufacturer must be certified and comply with ISO 13485: 2016 requirements (or equivalent).
Marketing approval certificate: Product and manufacturer must conform to European Medical Device Regulation (EU) 2017/745 (or equivalent).
Packaging and labelling:
Primary packaging: Each syringe and needle bi-packed in an individual sterilized peel-off pack made of paper and/or plastic.
Secondary packaging: Protective packaging - 1 carton of 100 bi-packed syringes with needles.
Related Products