S0481056
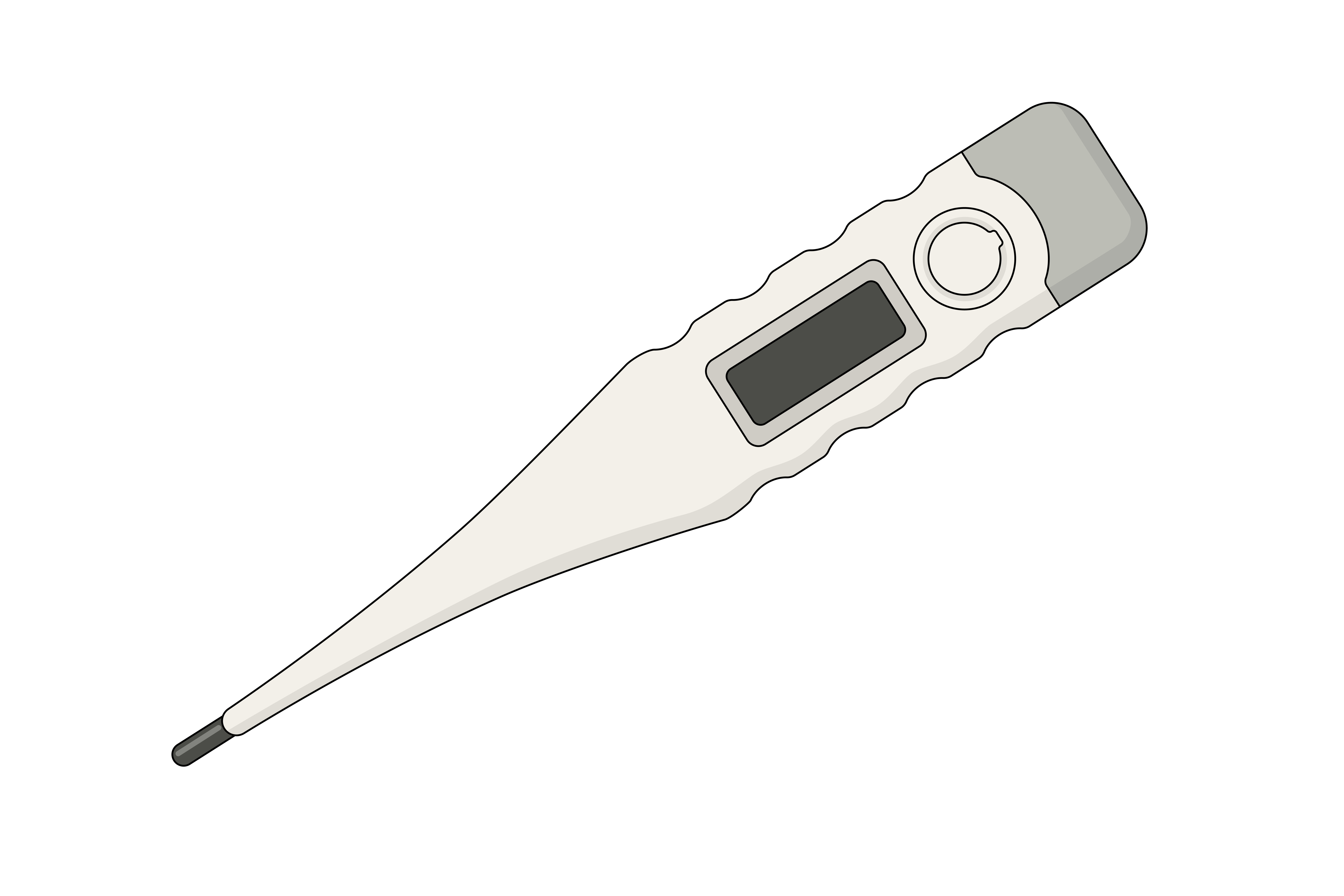
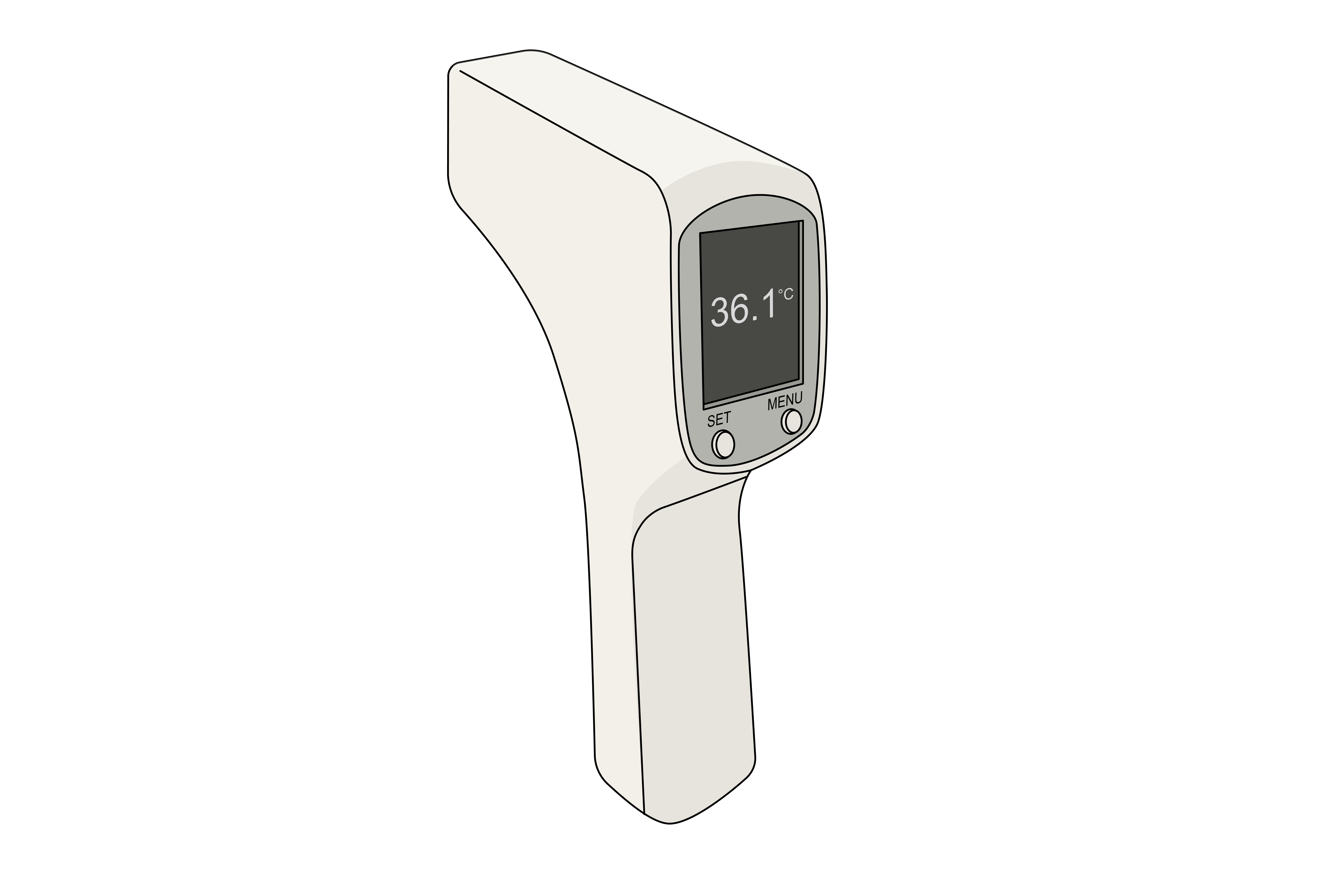
Thermometer,clinical,digital,waterproof
Clinical thermometer, digital, electronic, glass and mercury free, including battery.
Indicative Price 2.23 USD
GENERAL DESCRIPTION
Clinical thermometer used for taking body temperatures measurements from oral, armpit and rectum.
INTENDED USE
A hand-held, battery-powered, electronic instrument designed to measure a patient's body temperature. It comprises of an electronic unit that detects and converts the changes in temperature into variations of some electrical characteristic, e.g., resistance or voltage. These variations of the electrical characteristics are processed in the electronic circuits and in turn displayed, for a short period, as temperature readings. Thereafter the display will automatically turn off or go into standby mode. This is a reusable device.
TECHNICAL SPECIFICATIONS
Glass and mercury free.
Digital, electronic version.
Temperature measurement range 32 – 43 °C (minimum guaranteed).
Accuracy ± 0.1°C in the range 35 – 41 °C.
Graduation 0.3°C or better.
Ready-to-use after switch-on within 10 s.
Measurement time: within 120 seconds.
Low and high temperature indication.
Display easy to read in all levels of ambient light.
Automatic switch-off when not in use.
Beep audio alert when device is turned on/ready to use or when temperature measurement is complete.
Low battery indicator.
Full batteries allow for a minimum of 4,000 measurements.
Waterproof.
Designed to withstand frequent cleaning and disinfection with hospital-grade products.
Battery powered, batteries included in the supply, preferably packed separately.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
Supplied in rigid plastic protective case.
SHELF LIFE
Not applicable.
ESTIMATED LIFE SPAN
Five to seven years.
WARRANTY
One or two years depending on the model supplied.
ENVIRONMENTAL CONDITIONS
Storage conditions: -20 - 55°C / 85% RH.
Operating conditions: 10 - 40°C / 85% RH.
WEIGHT AND VOLUME (Packaged)
Weight: 40 gr.
Volume: 0.100 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
There are no installation or commissioning requirements.
TRAINING REQUIREMENTS
No user training will be required.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements.
RELATED PRODUCTS
S0481057, Thermometer,clinical,non-cntct,incl bat
COMPONENT OF A KIT
S9908305 - Obstetric,surgical kit,suppl.2-equipment
S9902420 - NBK,Community,Part B, Module 2,Equipment
S9902450 - NBK, Clinic, Module 3, Equipment
S9903002 - AWD, Periphery kit, Equipment
S9902221 - Midwifery kit – equipment, 2
S9901029 - IEHK2017,kit,suppl.2-equipment
S9901024 - IEHK2017, kit, basic unit
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDD 93/42/EEC as Class IIa device.
SAFETY AND PRODUCT STANDARDS
- ISO 80601-2-56: 2017: Medical electrical equipment - Part 2-56: Particular requirements for basic safety and essential performance of clinical thermometers for body temperature.
- ASTM E1112-00(2018) Standard specification for electronic thermometer for intermittent determination of patient temperature.
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- EN ISO 15223-1 (EN 980) Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements.
NOMENCLATURE
GMDN Code: 14035
Related Products