S0004316
Oxygen concentrator, 10 LPM, up to 1500m
Oxygen concentrator, 10 LPM – single flow, for elevation up to 1,500m.
Indicative Price 1,040.00 USD
GENERAL DESCRIPTION
A mains electricity powered device that concentrates oxygen from ambient air and delivers the concentrated oxygen in a controlled manner to a patient requiring oxygen therapy via a single outlet up to 10 L/min. This model is suitable in settings with elevation up to 1500m.
TECHNICAL SPECIFICATIONS
Features:
Provides a continuous, variable flow of concentrated oxygen up to 95.5% derived from room air.
Contains oxygen sensor to verify concentration.
Equipped with a single oxygen outlet, with a controllable flowmeter.
Continuous flow to a maximum rate of 10 L/min.
Minimum deliverable flow rate of 2 L/min.
Flowmeter continuously adjustable, with markings at 0.5L/min intervals.
Outlet pressure: 0.14 MPa (20 psig, 1.4 bar).
Power efficiency less than or equal to 70 W/L/min.
Noise level < 60 dB(A).
Display:
User interface easy to operate; numbers and displays clearly visible and easily readable in low ambient light and sunlight.
Digital meter that displays cumulative hours of device operation.
Alarms:
Audible and visual alarms for low oxygen concentration (85% ± 3%).
Audible and visual alarms for power supply failure.
Audible and visual alarms for high temperature.
Audible and visual alarms for low pressure.
Audible and visual alarms to indicate cannula flow blockage.
Casing and environment:
Hard case, cleanable according to manufacturer's instructions.
Oxygen outlet securely mounted and sheltered to reduce risk of being broken or bent.
The unit includes internally and externally mounted filters for cleaning the air intake.
All user-removable filters are cleanable. Cleaning instructions for filters are included in the instructions for use.
Whole unit movable with wheels on at least two castors.
Unit weight < 27 kg.
Electrical characteristics:
Mains power cable length greater than or equal to 2.5m.
Voltage: available in 230V/50Hz or 115V/60Hz.
Capacity for safe operation from at least ± 10% of rated voltage.
Electrical protection by resettable circuit breakers, fitted in both neutral and live lines.
SUPPLIED WITH
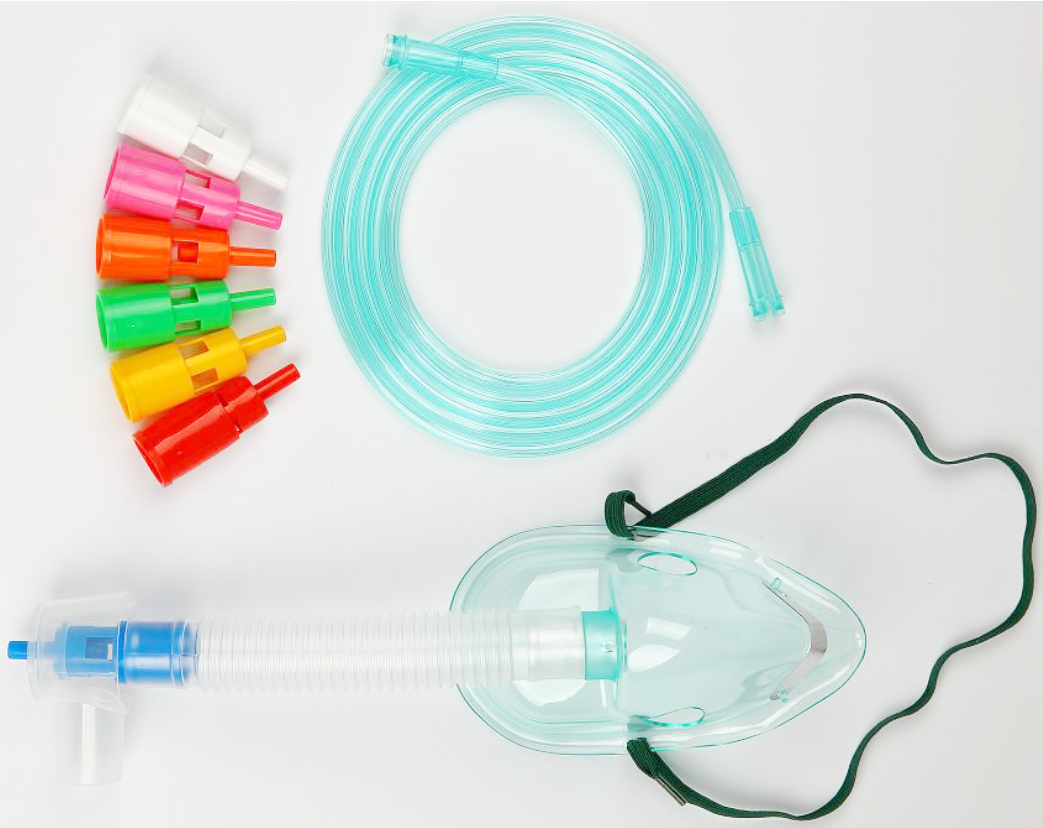
4 x adult nasal cannulas with 2m kink-resistant oxygen tubing with standard connectors.
4 x paediatric nasal cannulas with 2m kink-resistant oxygen tubing with standard connectors.
4 x neonatal nasal cannulas with 2m kink-resistant oxygen tubing with standard connectors.
1 x reusable humidifier bottle, up to 15LPM.
1 x DISS to 6mm barbed adaptor.
Internal and external filters for user fitting, as described in user manual, for 2 years' operation at 15 hours per day (in addition to those already included within the unit):
-- 1 x cabinet filter (PN 9250-1025).
-- 2 x air inlet filter (PN 9250-1180).
1 x set user and maintenance manuals in English, French and Spanish. If compete manuals are only available digitally, a quick reference guide and information sheet which clearly indicates the url to access complete manuals must be included with the device.
1 x certificate of calibration and inspection.
1 x list of all equipment and procedures required for routine maintenance.
1 x list of all spares and accessories, with part numbers and contact details for parts supply.
1 x document with contact details of manufacturer, supplier and local service agent.
ITEMS RECOMMENDED, BUT NOT SUPPLIED
Filter set (see Related Products); 1 filter set per concentrator is recommended.
Spare part kit (see Related Products); 1 spare part kit per 10 concentrators is recommended.
ESTIMATED LIFE SPAN
10 years.
Manufacturer commits to the availability of spare parts & consumables for the above indicated life span.
WARRANTY
Includes 2-year standard warranty, dated from the completion of the terms of delivery.
ENVIRONMENTAL CONDITIONS
Capable of being stored continuously in ambient temperature of -20°C to 60°C, relative humidity 15 to 95%.
Continuous operation within specification in ambient temperature of 10°C to 40°C, relative humidity 15 to 95% and altitude of up to 1500m.
IPX1 ingress protection.
WEIGHT/VOLUME (Packaged)
Weight: 30.9kg
Volume: 0.164m³
AFTER SALES AND TRAINING SERVICES
Spare parts and training services can be provided upon request.
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified for ISO 13485:2016 Medical devices. Quality management systems. Requirements for regulatory purposes.
REGULATION & CONFORMITY REQUIREMENTS
CE certificate as a class IIb medical device under EU regulation MDR 2017/745/EEC.
US FDA medical clearance (class II) - 510k.
SAFETY & PRODUCT STANDARDS
Complies with the following standards:
ISO 14971 Medical Devices - Application of risk management to medical devices.
ISO 80601-2-69: 2020 Medical electrical equipment - Part 2-69: Particular requirements for basic safety and essential performance of oxygen concentrator equipment.
IEC 60601-1 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
IEC 60601-1-2 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
IEC 60601-1-6 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability.
IEC 60601-1-8 Medical electrical equipment - Part 1-8: General requirements for basic safety and essential performance - Collateral standard: General requirements, tests and guidance for alarm systems in medical electrical equipment and medical electrical systems.
IEC 60601-1-11 Medical electrical equipment - Part 1-11: General requirements for basic safety and essential performance - Collateral standard: Requirements for medical electrical equipment and medical electrical systems used in the home health-care environment.
Related Products