S0845049
Nasal Catheter, 8 Fr
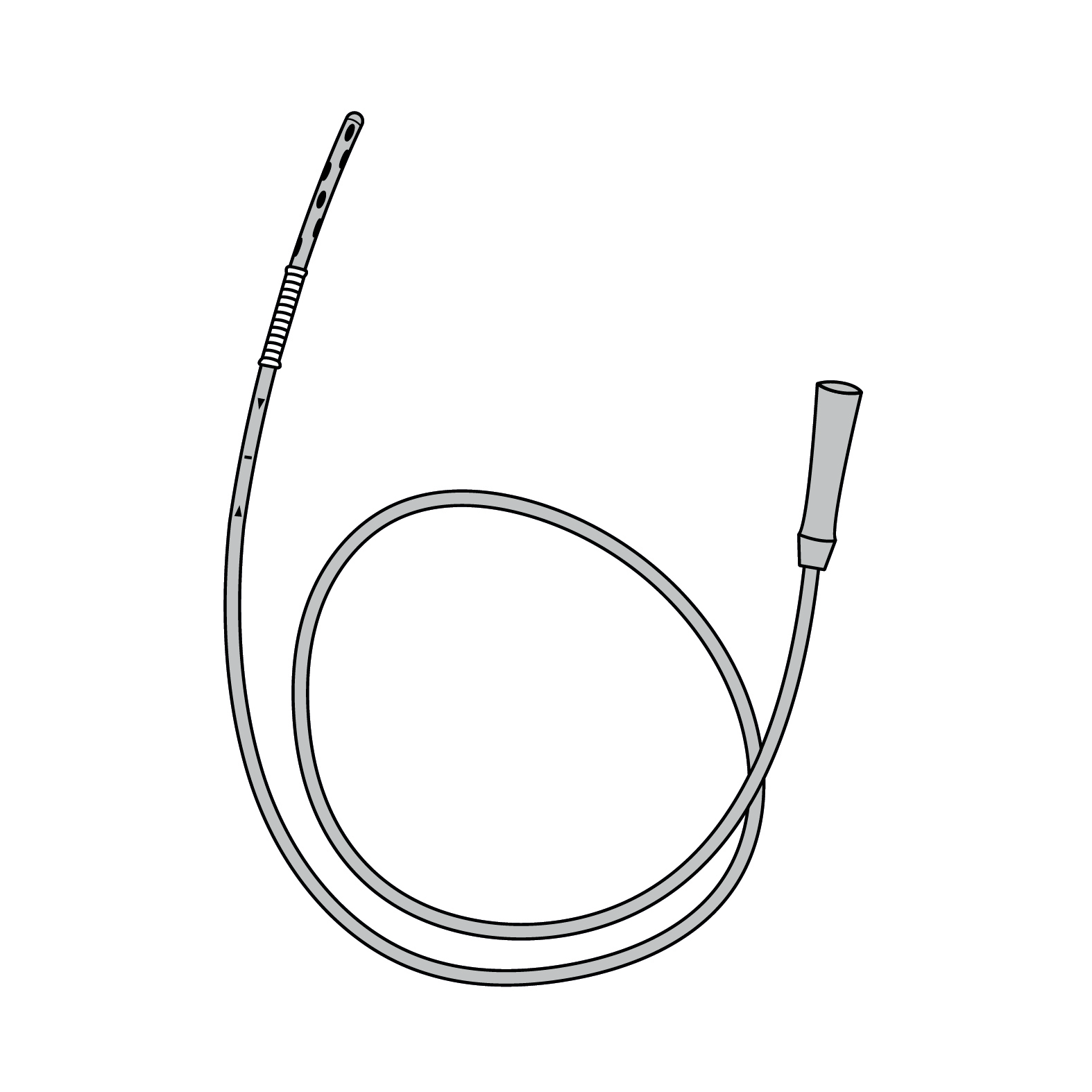
A flexible 8 Fr tube, typically lubricated with a water-soluble gel, that is inserted through a naris to deliver supplemental oxygen (O2) to the nasopharynx. This is a single-use device.
Indicative Price 0.28 USD
GENERAL DESCRIPTION
A flexible 8 Fr tube, typically lubricated with a water-soluble gel, that is inserted through a naris to deliver supplemental oxygen (O2) to the nasopharynx. This is a single-use device.
Minimum order quantity: 10,000 pieces if ordering direct from supplier, or single units from UNICEF warehouse.
TECHNICAL SPECIFICATIONS
Flexible nasal catheter with hole at distal end.
Consists of connector, tubing and sponge at distal end.
Oxygen and air/oxygen mixture compatibility, as per ISO 15001.
Transparent tubing, suitable for low pressure gas supply.
Rubber or soft plastic tubing and catheter, semi-rigid and allowing freedom of movement, PVC certified for medical use, hardness > 60 Shore A (ASTMD-2240).
8 Fr tubing, catheter length 40 cm.
Anti-kink tubing, non-permanent deformation if kinked or bent too tight.
Connecting tubing not included, should be ordered separately.
Single use, sterile product
ENVIRONMENTAL CONDITIONS
Operating conditions: 5 to 45°C, 15 to 90% relative humidity, non-condensing
Storage conditions (packaged): Store under cool & dry conditions. Do not expose to temperatures above 49°C.
ESTIMATED LEAD TIME
10 weeks
WEIGHT/VOLUME
Weight: 8.00 gr (each, packaged)
Volume: 0.132 dm3 (each, packaged)
WARRANTY
24 months
SHELF LIFE
60 months
REQUIRED BUT NOT SUPPLIED
S0845043, Tubing, oxygen supply, 7.6 mtr
RELATED PRODUCTS
S0845039, Flow splitter,for oxygen concentrator
S0845038, Oxygen concentrator, 10 LPM, single flow
S0845033, Oxygen concentrator, 10 LPM, dual flow
S0845213, Oxygen concentrator, 10 LPM, up to 1500m
S0845195, Oxygen concentrator, 8LPM, single flow
S0845008, Oxygen concentrator, 8LPM, double flow
S0845041, Oxygen concentrator, 5 LPM
ALTERNATIVE PRODUCTS
S0370115, Prongs,nasal,Oxygen,child,s.u.
S0370125, Prongs,nasal,Oxygen,neonate,s.u.
CLASSIFICATION
93/42/EEC Risk class IIa or higher– CE certificate
SAFETY & PRODUCT STANDARDS
Complies with the following standards:
ISO 15001 Anaesthetic and respiratory equipment — Compatibility with oxygen
ISO 13485:2003 Medical devices -Quality management systems --Requirements for regulatory purposes
PACKAGING / LABELLING
Sterile
Labelling on primary packaging must include:
- Name and/or trademark of the manufacturer
- Manufacturer's product reference
- Type of product and main characteristics
- If the packaging is not transparent, it must bear a diagram showing the essential parts of the product
- Information for particular storage conditions (temperature, pressure, light, humidity, etc.), as appropriate (or equivalent harmonised symbol)
- Information for handling, if applicable (or equivalent harmonised symbol)
Over packaging: Packaging unit
Labelling on the packaging unit: Labelling to be the same as primary packaging
Number of units per box: (100 pieces in a bag) 600 pieces in a carton.
Related Products