S0002632
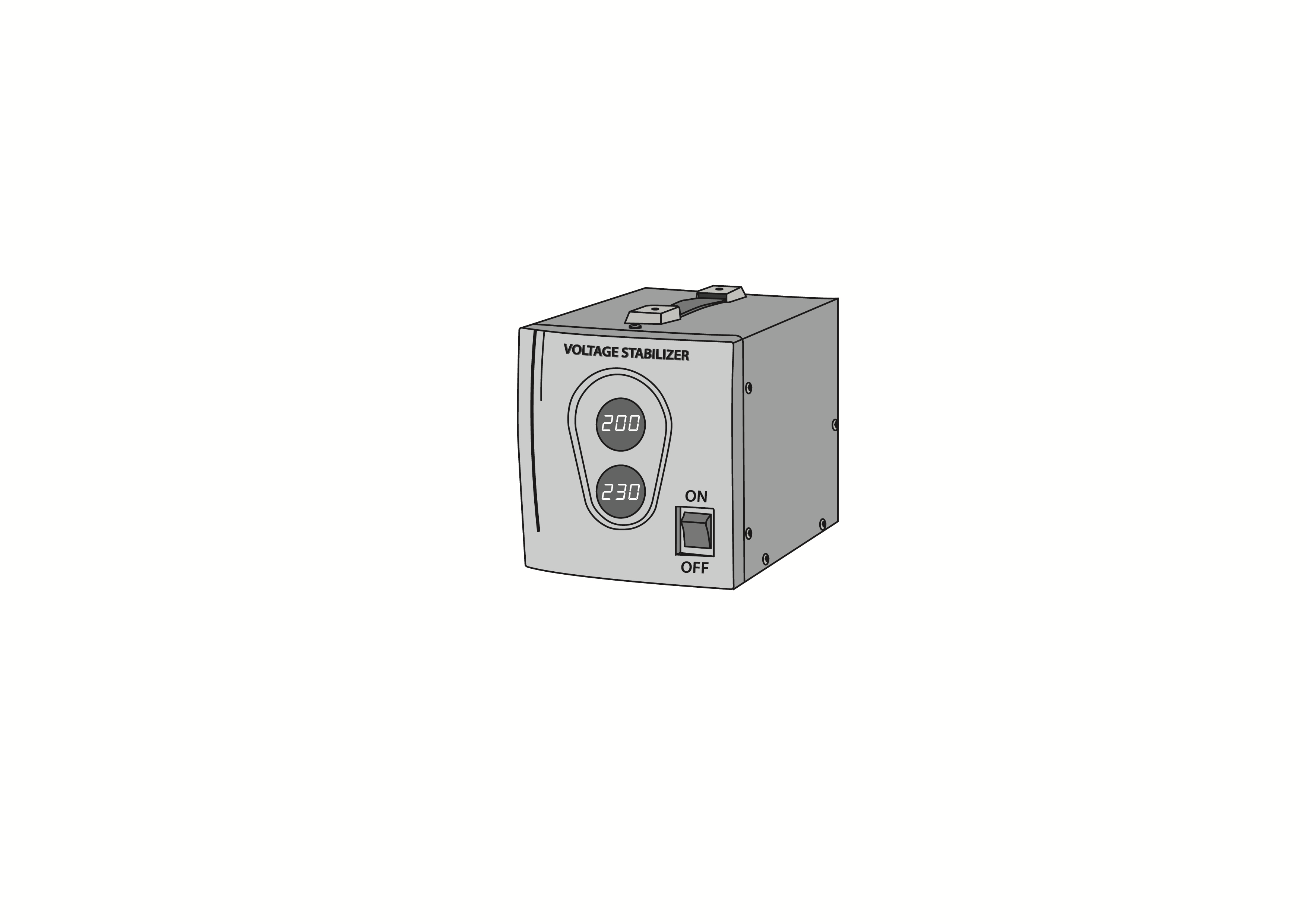
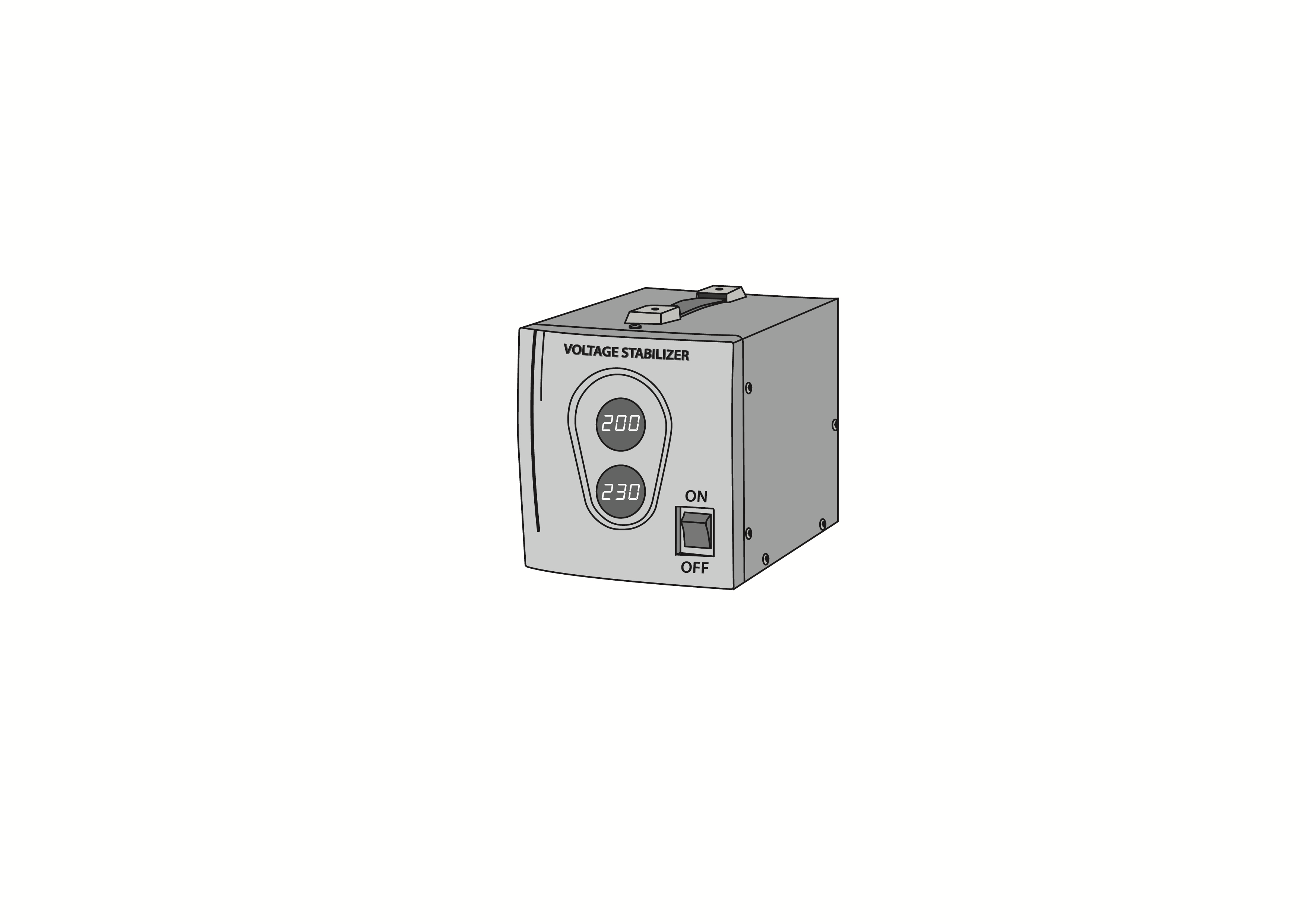
Voltage stabilizer, 1 kVA
Voltage stabilizer, 1kVA load rating, single phase, 230V 50/60Hz. Reduces fluctuations in input voltage for medical equipment. Not for use with refrigerators and freezers.
Indicative Price 151.61 USD
GENERAL DESCRIPTION
Voltage stabilizer installed between the mains power and the electrical medical equipment to stabilize the mains voltage to a pre-determined output, eliminating its fluctuations and protecting the electrical equipment from surges. Load rating 1kVA. 230V 50/60Hz model.
TECHNICAL SPECIFICATIONS
Stabilization:
Electronic, microprocessor-controlled voltage stabilizer.
Solid state tap-switching technology.
Single phase.
Load rating 1 kVA.
At least 173 – 264 VAC nominal input voltage, or better.
Frequency at least 50-60Hz.
Output voltage: 202V to 245V, or better.
One (or two) output socket(s). If two output sockets are required, please specify at time of ordering.
Voltage input and output indicators.
Suitable for short term overload working conditions, at least 135% for 4 mins and 110% for 6 mins.
Efficiency of at least 80% at 25% load, 90% at 50% load and 95% at 75-100% load.
Correction speed ≥ 750 V/s.
Unaffected by load power factor.
Low noise ≤ 45dB.
Protection:
Input resettable circuit breaker for overload or short circuit.
Spike, surge, and transient protection.
Automatic over-voltage and under-voltage protection, with output power supply disconnection.
Automatic start-up after disconnection or power failure, with start-up delay 10 ± 2 seconds.
Protection of the regulator and load against switch on/off over current and over voltage.
Visual alarm for operational conditions and system status.
Indoor operation, enclosure protection at least IP20.
Suitable for use on grounded and non-grounded mains, with the possibility to use an alternative grounding.
Power cord length ≥1.5 m.
Plug type E or F.
Other output sockets and plug types may be available (MOQ >200 may apply for custom plug type requests). Please specify if a different plug type is required at the time of ordering.
ENVIRONMENTAL CONDITIONS
Capable of being stored in ambient temperature of at least -5 to +50°C, relative humidity at least 10 to 95% non-condensing.
Capable of continuous operation in ambient temperature of at least -5 to +50°C, relative humidity at least of 10 to 95% non-condensing.
SUPPLIED WITH
1 x Set user and maintenance manuals to be supplied in English, French and Spanish. If manuals are only available digitally, a quick reference guide and information sheet which clearly indicates the URL to access complete manuals to be included with the device.
1 x Label on equipment stating that stabilizer is not for use with cold chain equipment.
Contact details of manufacturer available in User Manual and/or website.
WARRANTY
Includes 2-year standard warranty, dated from the completion of the terms of delivery.
SHELF LIFE
N/A
ESTIMATED LIFE SPAN
At least 10 years.
WEIGHT/VOLUME
Weight: ~7.85kg
Volume: ~0.025m³
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified to ISO 9001 Quality management systems -- Requirements.
REGULATION & CONFORMITY REQUIREMENTS
Conforms to the essential requirements of the European Low Voltage Directive (2014/35/EU) - CE Declaration of Conformity.
SAFETY & PRODUCT STANDARDS
Complies with at least the following harmonized standards:
EN 61558-1:2005+A1:2009 (or later version) - Safety of power transformers, power supplies, reactors and similar products - General requirements and tests.
EN 61558-2-13:2009 Safety of transformers, reactors, power supply units and similar products for supply voltages up to 1100 V - Particular requirements and tests for auto transformers and power supply units incorporating auto transformers.
EN 61000-3-2 Electromagnetic compatibility (EMC) - Limits. Limits for harmonic current emissions (equipment input current ≤16 A per phase).
EN 61000-3-3:2013 Electromagnetic compatibility (EMC) - Limits. Limitation of voltage changes, voltage fluctuations and flicker in public low-voltage supply systems, for equipment with rated current ≤ 16 A per phase and not subject to conditional connection.
PACKAGING / LABELLING
Packing and labelling in compliance with the requirements as set out on the UNICEF Supply website under this hyperlink: https://www.unicef.org/supply/technical-specifi cations-packing-packaging-and-labelling
Related Products