S0370130
Face mask, venturi, adult, single use
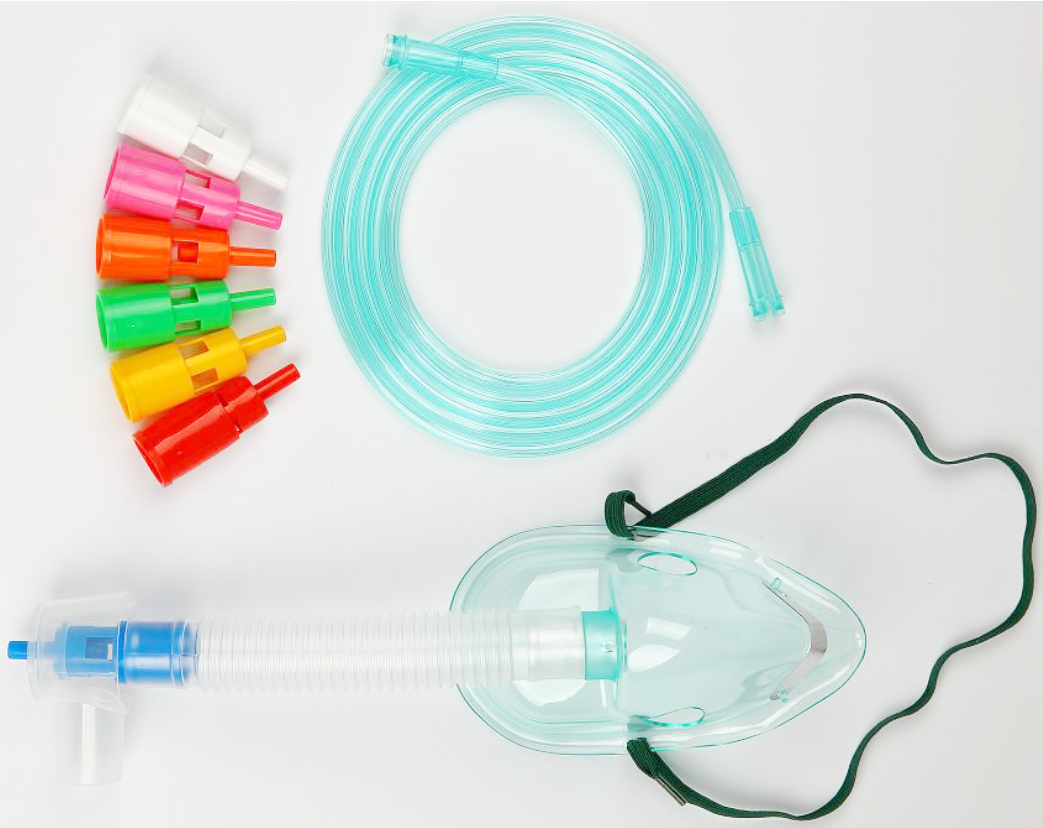
Face mask to provide oxygen at flow rates up to 12 LPM. Allows for precise oxygen delivery by utilizing different sized ports to change FiO2 (24 – 60%). Also known as air-entrainment masks able to provide total inspiratory flow at a specified FiO2. Adult size.
Indicative Price 1.13 USD
GENERAL DESCRIPTION
Face mask to provide oxygen at flow rates up to 12 LPM. Allows for precise oxygen delivery by utilizing different sized ports to change FiO2 (24 – 60%). Also known as air-entrainment masks able to provide total inspiratory flow at a specified FiO2.
TECHNICAL SPECIFICATIONS
Allows for precise oxygen delivery, with specific concentrations ranging from 24-60%.
Mask is soft, transparent, with well-fitting mould.
The nose clip is adjustable.
The kit of the mask includes the tubing, humidity cup and multiple jets, which are color-coded and indicate the percentage of oxygen.
Tubing compatible with standard oxygen connection tubing.
Tubing length: 2 meters
The tubing is terminated with standard or universal hose end connector.
Individually packed.
Non-sterile.
Single use.
Adult size.
Material: Mask and tubing PVC or other material, FDA Title 21/USP VI compliant and certified for medical use, hardness > 60 Shore A (ASTM D-2240).
ENVIRONMENTAL CONDITIONS
Operating conditions: 5 to 40 deg. C, 10 to 85% relative humidity
Storage conditions: 0 to 60 deg. C, 10 to 90% relative humidity
ESTIMATED LEAD TIME
4-6 weeks
WEIGHT/VOLUME
Weight: 0.12kg (each, packaged)
Volume: 0.001008m3 (each, packaged)
SHELF LIFE
5 years
ALTERNATIVE PRODUCTS
S0370105, Prongs,nasal,Oxygen,adult,s.u.
S0370115, Prongs,nasal,Oxygen,child,s.u.
S0370125, Prongs,nasal,Oxygen,neonate,s.u.
REGULATION & CONFORMITY REQUIREMENTS
CE mark conforming to Medical Device Directive 93/42/EEC
CE Certificate (class IIa or higher)
CLASSIFICATION
93/42/EEC Risk class IIa or higher– CE certificate
SAFETY & PRODUCT STANDARDS
Must comply with the following standards:
ISO 13485:2003 Medical devices -Quality management systems -- Requirements for regulatory purposes
ISO 15001:2010 Anaesthetic and respiratory equipment – Compatibility with oxygen.
ISO 10993:2018 Biological evaluation of medical devices
BS EN 13544-2:2002+A1:2009 Respiratory therapy equipment. Tubing and connectors
PACKAGING / LABELLING
Unit presentation: 1 (one)
Number of units per box: 100 or 50 pieces (depending on manufacturer)
Related Products