S0002063
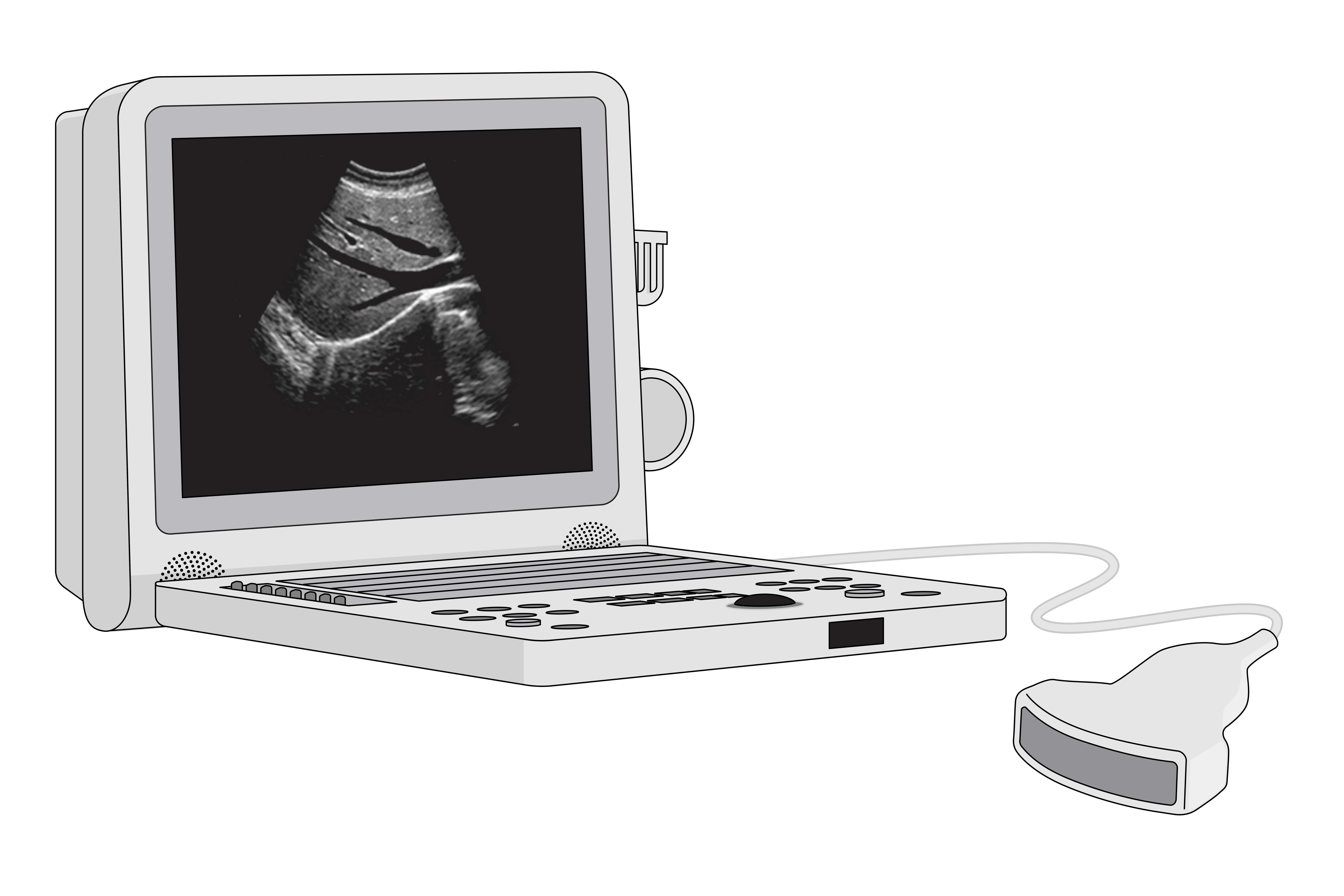
Scanner,ultrasound,mobile,w/access
Scanning system, ultrasonic, portable, for OB/GYN, abdomen, small parts, cardiology, urology and generic diagnostics, with convex and transvaginal probes, trolley and accessories.
Indicative Price 2,888.58 USD
GENERAL DESCRIPTION
General purpose, portable ultrasound scanner, with obstetrics package and probes.
INTENDED USE
Portable ultrasonic scanning systems are used in physician offices, radiology departments, cardiology departments, emergency departments, critical care units and by mobile imaging service providers. They can also have important applications in disaster triage and mass casualty incidents. The main benefit of point of care ultrasounds units is that it allows physicians to quickly determine whether an abnormality is present so they can make appropriate patient management decisions.
TECHNICAL SPECIFICATIONS
General purpose ultrasound scanner, with obstetrics package and probes.
System includes a trolley.
The system supports B mode and M mode.
The unit includes: one (1) convex array probe, 2.5-5 MHz and one (1) endovaginal probe, 5.5-7.5 MHz.
The unit supports user programmable exams (exam pre-sets).
An obstetrics analysis package is included which provides for foetal weight and anatomy, gestational age and foetal growth calculations.
The unit comes standard with packages for: abdomen, gynaecology/obstetrics, cardiology and small parts.
Depending on the model supplied Pulsed Wave Doppler (back/white), is included in the unit.
Power requirements: 100 - 240 Volts - 50/60 Hz (not necessarily in a single unit).
IMAGE DISPLAY
Digital callipers.
Cine loop.
A minimum of 4 x zoom.
256 levels of greyscale.
A freeze function.
Capable of lateral and vertical inversion in B mode.
Capable of displaying B+M modes simultaneously.
Capable of displaying 2B and 4B modes.
Full screen annotation, for real-time and frozen images.
COMPONENTS
Time gain control (TGC).
Tissue harmonic imaging.
Optional B-mode optimization.
The unit has a range of pre- and post-processing fetures to enhance images and automated image optimization.
A black and white display with a minimum of 25 cm (9.7 inch) diagonally.
Equipped with an alphanumeric keyboard and a trackball.
Two probe ports can be connected simultaneously, machine selectable from interface.
CART/TROLLEY
Includes at least two side probe holders.
Side handle(s) for transport and positioning.
Surface space for scanner and keyboard.
Storage space (shelf) for printer.
Four anti-static braking swivel castors, at least 10 cm high.
DATA STORAGE AND COMMUNICATIONS
Internal hard drive for limited data storage.
Supported communication protocols: USB, S-video, VGA, and depending on the model: RS 232 or ethernet).
Supported formats for image export: JPEG.
PERFORMANCE AND SAFETY
Self-test is performed each time the machine is switched on.
System reports, with audio-visual alert on: operational status and malfunctions.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish:
1 x Plastic protective dustcover.
3 x bottle of 100 ml (or 2 X 150 ml) of ultrasound gel.
1 x Set of spare fuses.
OPTIONAL ACCESSORIES AVAILABLE
At extra costs additional optional features can be requested:
- A linear array probe 5.5- 8.5 MHz can be added to the unit.
- A micro-convex probe, 5.0 -7.5 MHz can be included in the unit.
- A rechargeable battery (standard in one model, optional in other model).
- DICOM 3.0 compatibility (Included standard in one model, optional in other model).
- A thermal printer, supporting a 256 greyscale, 325 dpi resolution at a minimum.
ESTIMATED LIFE SPAN
Five years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 5°C – 40°C / 25% - 80% RH.
- Storage conditions: -20°C - 55°C / 25% - 93% RH.
- Atmospheric pressure: 860 ~ 1060 hPa.
WEIGHT AND VOLUME (packaged)
Weight: 118.00 kg.
Volume: 511.00 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
This product does not have complex assembly and/or commissioning needs requiring a technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIa device
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-2-37:2007+AMD1:2015 Medical electrical equipment - Part 2-37: Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment.
NOMENCLATURE
- GMDN code: General-purpose ultrasound imaging system (40761).
- UMDNS code: Scanning Systems, Ultrasonic, General-Purpose (15976).
Related Products