S0002100
Nebulizer,mesh,handheld,w/ access
Nebulizer, mesh type, handheld, battery powered, with accessories.
Indicative Price 20.10 USD
GENERAL DESCRIPTION
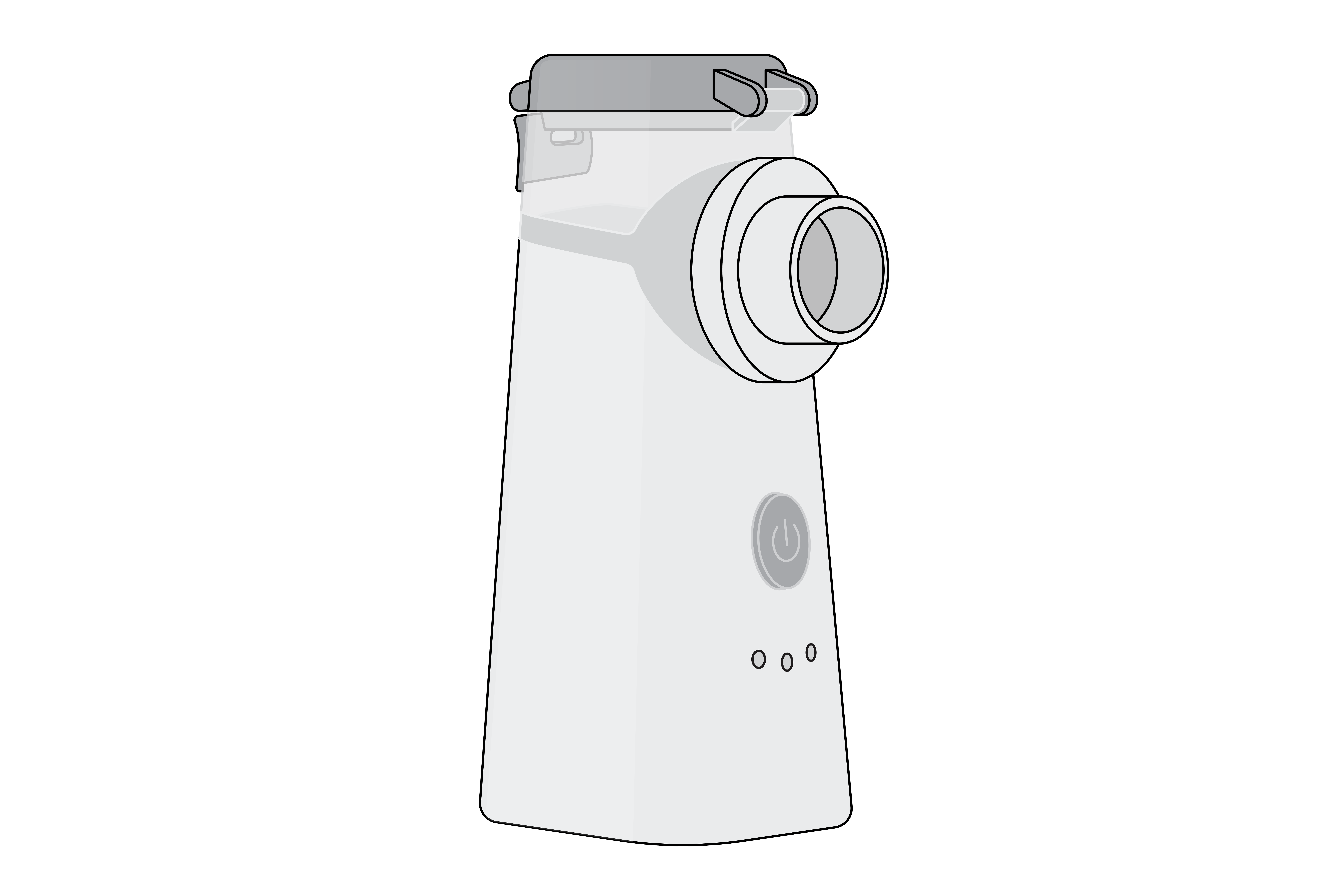
A handheld nebulizer using a vibrating mesh, battery powered, with accessories.
Note: Not suitable to be used in conjunction with other respiratory support equipment such as: ventilators, anaesthesia machines, CPAP devices, etc.
INTENDED USE
A device designed to generate aerosolized medication/fluids (finely dispersed airborne droplets) for inhalation by a patient with a respiratory disorder, for example chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF).
TECHNICAL SPECIFICATIONS
Nebuliser for the treatment of upper/lower respiratory system and for the delivery of nebulised drugs.
The unit uses vibrating mesh technology.
A handheld model.
Will auto switch off after 20 minutes but allows for immediately restart.
Minimum drug nebulisation flow greater than: 0.2 ml/min.
Integrated drug reservoir with a minimum capacity: 12 ml.
Residual volume (not operating below) less than 0.5 ml.
Median Mass Aerodynamic Diameter (MMAD) of particles 2.3 µm (±25%).
Noise level ≤ 50 dB
Sturdy construction, suitable to be disinfected with hospital-grade products.
Power requirements: 2 x AA sized alkaline batteries
SUPPLIED WITH
Instructions for assembly, use and maintenance in English.
1 x carry bag
2 x reusable mouth-piece
4 x reusable adult face masks
4 x reusable paediatric face masks
ESTIMATED LIFE SPAN
2 years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 10°C - 40°C / 10% – 80% RH.
- Storage conditions: -20°C - 70°C / 10% – 95% RH.
- Atmospheric pressure: 500 ~ 1060 hPa.
- Ingress protection rating: IP21.
WEIGHT AND VOLUME
Weight: 110 gr kg.
Volume: 0.357 dm³.
ESTIMATED DELIVERY LEAD TIME
100 days.
INSTALLATION REQUIREMENTS
No installation required.
TRAINING REQUIREMENTS
User training is not required.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified under EU MDD 93/42/EEC as Class IIa device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- EN 13544-1:2007 Respiratory therapy equipment - Part 1: Nebulizing systems and their components.
NOMENCLATURE
- GMDN© code: 66295
- EMDN: R0601
Related Products