S0002644
Bilirubin meter,transcutaneous,hand-held
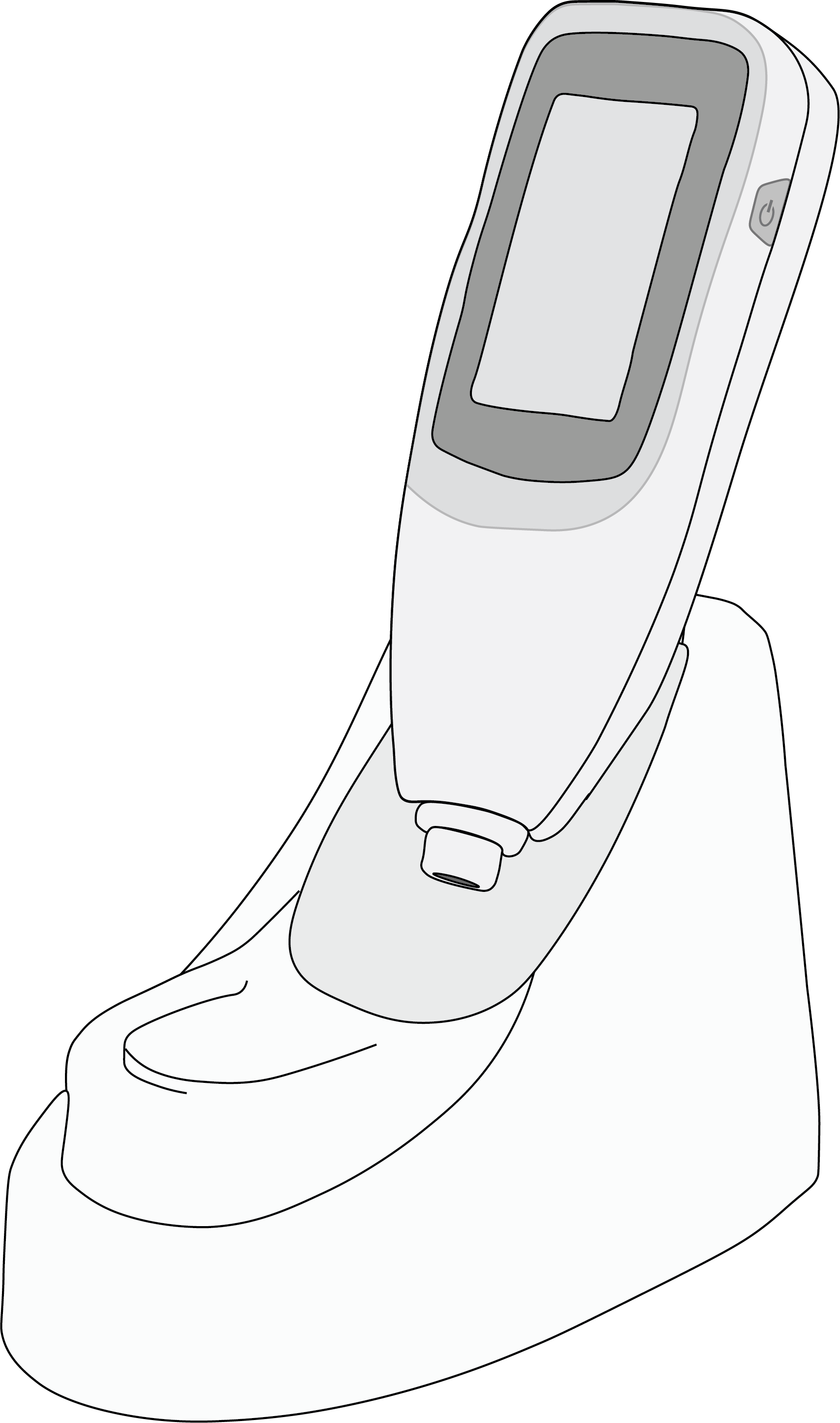
The transcutaneous bilirubin meter uses a non-invasive method to measure transcutaneous bilirubin level which allows to predict total serum bilirubin levels in a newborn. The hand-held device is battery operated.
Indicative Price 4,941.18 USD
GENERAL DESCRIPTION
A noninvasive, battery-powered, hand-held, portable instrument intended to measure transcutaneous bilirubin (TcB) levels as a method of predicting total serum bilirubin (TSB) and screening for physiologic jaundice in neonates. It directs a light beam into the newborn’s skin, usually on the forehead, sternum or ear, and measures the intensity of multiple wavelengths (typically 2 or more) that are reflected from the newborns subcutaneous tissue. These optical signals are processed (typically by a photocell and microprocessor) to give a TcB value.
INTENDED USE
Physiologic neonatal hyperbilirubinemia or jaundice occurs in almost all new-born patients. Neonatal jaundice is caused by increased levels of bilirubin, a product of haemoglobin breakdown. If left untreated, jaundice may cause kernicterus (a condition where high levels of bilirubin cross the blood/brain barrier) which can cause permanent brain damage and long-term developmental disorders. A transcutaneous bilirubinometer is a non-invasive point-of-care device that provides an estimate of total serum bilirubin. This reusable product is battery operated and can be used to quickly screen multiple patients for jaundice. Patients may then be further tested using a serum bilirubinometer for exact values of TSB before being started on phototherapy or recommended for exchange transfusion. Transcutaneous bilirubinometers provide a convenient and painless alternative to blood sampling for screening purposes, reducing the need for frequent blood tests in newborns.
TARGET POPULATION
Newborn and premature babies and infants.
TECHNICAL SPECIFICATIONS
A portable non-invasive transcutaneous bilirubin meter that measures yellowness of subcutaneous tissue to accurately and precisely estimate serum bilirubin using a xenon lamp or LED absorbance system with two optical paths towards two set of photodiodes.
For use by clinically trained caregivers as a screening mechanism to determine physiologic jaundice in newborns (> 24 weeks gestational age who have not undergone exchange transfusion) and infants of all skin tones from 0 to 14 days of age within a health facility.
Portable assembled product weighing not more than 200g, ensuring easy transport and care for the newborn patient.
Transcutaneous bilirubin measurement provided in a quantitative format to estimate Total Bilirubin, displayed in mg/dL or µmol/L with ability to select units and switch between units on the same device.
Samples taken on the infant's forehead, sternum or ear on intact skin. Not indicated for use on bruised skin, birthmarks or other skin lesions.
Sampling range 0 to 20 mg/dL, readings above 20mg/dL provoke a “High Bilirubin Level” alert message or similar.
Repeatability (SD) 0.7mg/dL and accuracy (RMSE) +/-1.5 mg/dL at 66% of the time or 1 standard deviation.
No consumables required for repeated use.
User conducted performance check available with checker ports on docking station.
Automatic diagnostic self-test on startup.
Display languages available in English, German, Spanish, French, Russian, Portuguese, and others.
CONSTRUCTION AND DESIGN
Transcutaneous Bilirubinometer
Unit displays results digitally on a screen and provides feedback via an LCD touchscreen, with options for performing a measurement, viewing results of historic measurements, user-performed check to confirm the device is correctly calibrated for use, and menu or settings access to set values for system parameters such as sound volume, screen brightness, etc.
Built-in barcode reader and manual entry with an onscreen keyboard for patient and nursing staff ID numbers.
Pulse xenon arc lamp or LED light source with silicon photodiodes.
Durable housing material designed to withstand frequent disinfection with hospital-grade products (e.g., mild soap and water solution, ethyl alcohol (70-85%) or isopropyl alcohol (IPA), diluted chlorine).
Memory sufficient to store 100 patient readings.
Docking Station
Power requirements 100 V~ to 240 V~, 50/60 Hz.
LED light indicators to confirm battery is charging and that charging stand is correctly connected to the power supply.
Connectivity to PC and HIS via USB and HL7 protocol via PC, respectively.
Battery
NiMH or lithium-ion battery allowing >250 measurements per full charge.
Maximum recharge time not more than 4 hours.
ERROR INDICATORS
Transcutaneous bilirubinometer alarms are both audible and visual. Errors range from sampling to device memory failures.
Measurement failure indicator with error defined on screen, including abnormal readings and readings out of measurement threshold.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x plastic protective dustcover.
1 x transcutaneous bilirubinometer.
1 x docking station and AC adapter.
SHELF LIFE
No limitations on product as provided. Spare battery packs if procured have a shelf life of 90 days.
ESTIMATED LIFE SPAN
Bilirubin measuring device: 5 years.
Internal battery: 1 year.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
Operating conditions: 10°C to 34°C / ≤ 75% RH.
Storage conditions: -20°C to 60°C / ≤ 90% RH.
Atmospheric pressure: 50 kPa to 106 kPa.
Ingress protection rating: IP20.
WEIGHT AND VOLUME (packaged)
Weight: 2.63 kg or less
Volume: 25.26 dm3 or less
MATERIAL SAFETY DATASHEET (MSDS)
Required.
DANGEROUS GOODS
UN 3481/3496.
UTILITY REQUIREMENTS
Intermittent supply of electricity (110-230V, 50/60 Hz, 130W/550W) as needed to charge the internal battery pack.
ESTIMATED DELIVERY LEAD TIME
42 Days
INSTALLATION REQUIREMENTS
Assembly and commissioning this product by a qualified technician is recommended.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals and standard protocols for infection prevention and control. Depending on the model supplied, yearly calibration at a manufacturer-approved calibration center might be a requirement.
RELATED PRODUCTS:
S0002018, Phototherapy irradiance meter
S0002032, Photo therapy unit,w/access
LABELLING
PRIMARY PACKING
In accordance with EU MDR 2017/745 Annex I, Chapter III, point 23.2. Follow below hyperlink for details on this regulation.
https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32017R074 5&qid=1622012817907&from=EN#d1e32-94-1
SECONDARY PACKING
- Labelling on the packaging unit: Labelling to be the same as primary packaging.
- Extra information required: Number of units per box.
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
Manufacturer is certified for ISO 9001 Quality management systems – Requirements.
REGULATION, CONFORMITY REQUIREMENTS & CLASSIFICATION
Classified under EU MDR 2017/745 as Class Im or IIa device.
SAFETY & PRODUCT STANDARDS
IEC 60601-1: Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
IEC 60601-1-2: Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
IEC 62304: Medical device software — Software life cycle processes.
IEC 62366-1: Medical devices — Part 1: Application of usability engineering to medical devices.
Related Products