S0002651
Consumables, CPAP,6mos,Phoenix
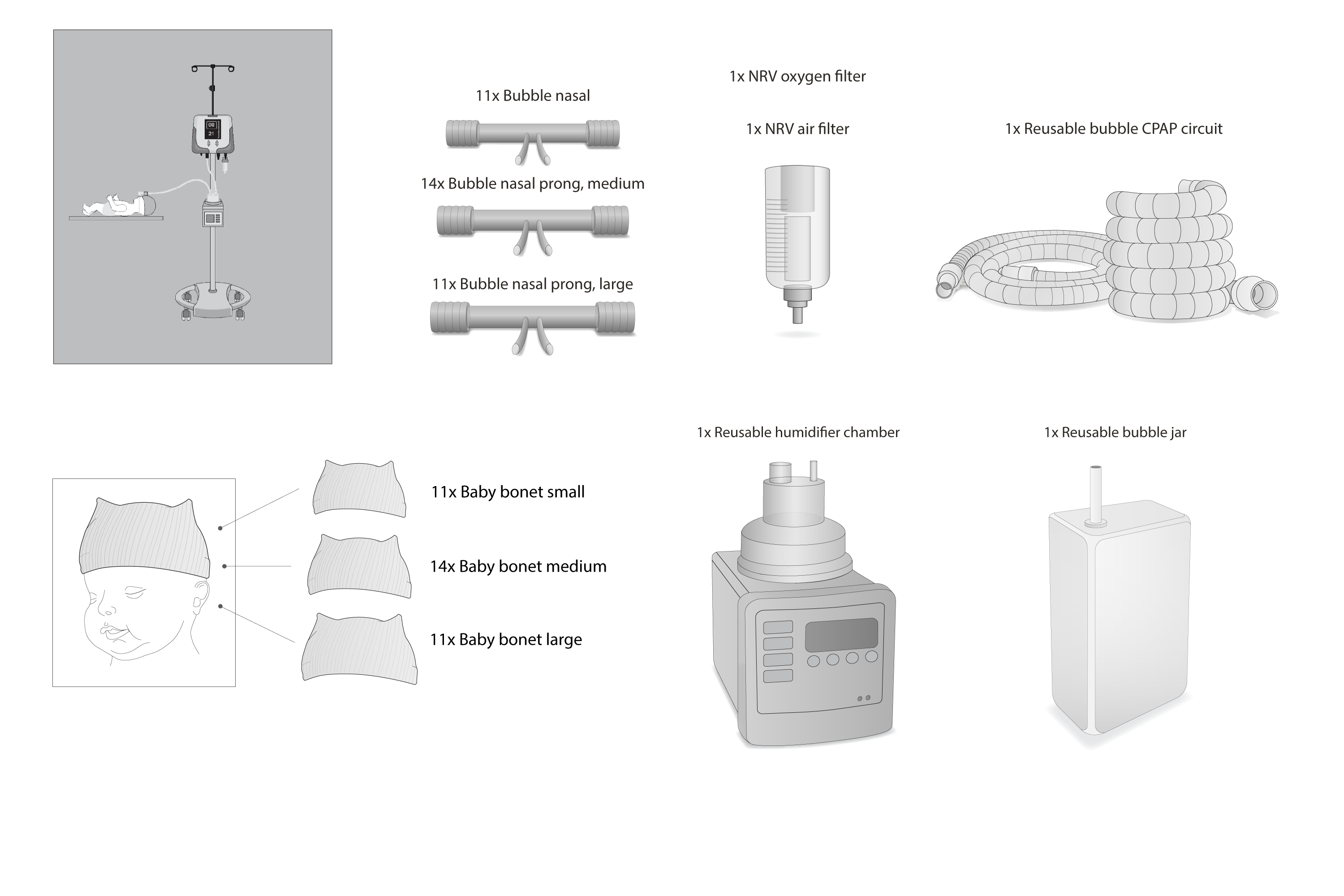
Consumables bundle for the Phoenix nCPAP 300. Bundle is for one CPAP device for an estimated 6 months of use, assuming a 5-day patient stay. This bundle contains nasal cannulas to support neonatal CPAP patients with weights ranging from 700 grams to greater than 2000 grams.
Indicative Price 2,137.59 USD
GENERAL DESCRIPTION
Consumables bundle for the Phoenix nCPAP 300. Bundle is for one CPAP device for an estimated 6 months of use, assuming a 5-day patient stay. This bundle contains nasal cannulas to support neonatal CPAP patients with weights ranging from 700 grams to greater than 2000 grams.
TECHNICAL SPECIFICATIONS
11 x Bubble nasal prong – small (PMS-Y-061) for newborns between 700 – 1250 grams
14 x Bubble nasal prong – medium (PMS-Y-062) for newborns between 1250 – 2000 grams
11 x Bubble nasal prong – large (PMS-Y-063) for newborns greater than 2000 grams
11 x Bonnet – small (bubble type) (PMS-Y-025)
14 x Bonnet – medium (bubble type) (PMS-Y-026)
11 x Bonnet – large (bubble type) (PMS-Y-027)
1 x Reusable bubble CPAP circuit (PMS-YR3)
1 x Reusable humidifier chamber (2010018)
1 x Reusable bubble jar (30230089)
1 x NRV filter – air (NRV/AIR)
1 x NRV filter – oxygen (NRV/O2)
STORAGE CONDITIONS
Temperature: 5°C - 40°C
Relative Humidity: 10% – 95%
WARRANTY
Includes 2-year standard warranty, dated from the completion of the terms of delivery.
SHELF LIFE
24 months
WEIGHT/VOLUME
Weight: 6kg
Volume: 0.061m³
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices – Quality management systems – Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
REGULATION & CONFORMITY REQUIREMENTS
Medical consumables have CE certificate under EU MDD 93/42/ECC or EU MDR 2017/745.
Related Products