S0004002
CPAP,newborn,w/compressor & O2 concentr
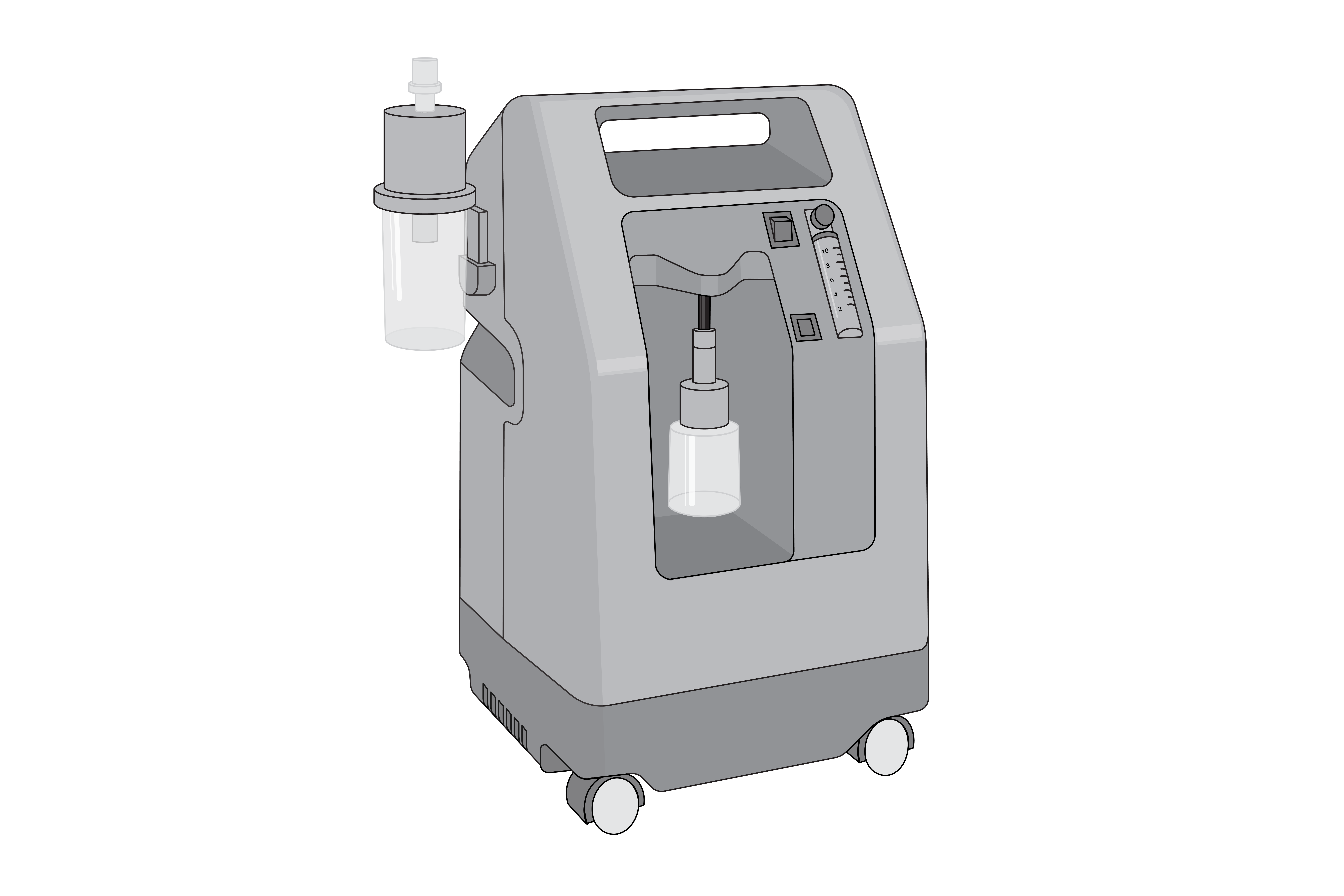
Bubble CPAP non-invasive respiratory support system complete system including internal air compressor and oxygen concentrator. Suitable for all normal, premature and low weight neonates up to 10kg. Supplied including humidifier, air/oxygen mixer and acces
Indicative Price 5,182.66 USD
IMPORTANT
Training and installation are not included with the device and must be requested separately from the supplier or manufacturer. Improper operation or maintenance can result in harm to patients and/or operators, including death. Therefore, UNICEF strongly recommends the inclusion of installation and training services for both operators and technicians, unless identical models of these devices are already in use at the intended installation site. Neither UNICEF nor the manufacturer can assume responsibility for any consequences arising from incorrect operation and/or maintenance. Training and installation will have to be quoted separately; the number of operators and technicians to be trained, and the locations where these trainings will have to take place, are required in order to obtain a quotation.
INTENDED USE
An electrically powered device designed to deliver high-flow (exceeding peak inspiratory flow) heated and humidified ambient air or air/oxygen to a neonatal patient as part of non-invasive ventilation (NIV); it may additionally be used with a water tank to produce bubble continuous positive airway pressure (bubble CPAP). It consists of a gas flow generator that entrains room air, an oxygen concentrator, a heating provision, and humidification chamber; it does not include CPAP controlling pressure sensors (i.e., not a full CPAP unit). It is intended for use by a healthcare provider on a spontaneously breathing patient in hospital settings.
SPECIFICATIONS
Medical bubble CPAP unit on 4 antistatic castors,
Provides continuous positive airway pressure to spontaneously breathing infants requiring respiratory support, for premature neonates and infants weighing up to 10kg.
Allows for the provision of warmed and humidified air to the patient.
Maximum total blended gas flow: 20L/min.
Maximum oxygen flow capacity: 10L/min.
Maximum air flow capacity: 10L/min.
Provides oxygen concentration in the range of 21% - 95%.
Supports pressures between 1 – 10 CmH2O through bubble controller.
Does not require external supply of compressed gas or oxygen due to internal compressor and oxygen concentrator.
Suitable for heated and non-heated closed patient circuits.
Including alarms for: power failure, low oxygen concentration, high and low pressure, no flow.
Supplied including voltage protector.
Power supply: 240VAC, 50Hz.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
- 2x Humidifier bottle.
- 1x Bubble pressure controller.
- 4x reusable patient circuits.
- 2 sets of extra small size reusable CPAP head bonnets.
- 2 sets of small size reusable CPAP head bonnets.
- 2 sets of medium size reusable CPAP head bonnets.
- 10x nasal cannula soft and atraumatic - extra small (for premature neonates).
- 10x nasal cannula soft and atraumatic - small for term and older infants.
- 1x plastic protective dustcover.
ESTIMATED LIFE SPAN
8 years.
WARRANTY
Two years from shipping date.
ENVIRONTAL CONDITIONS
Storage conditions: -20 - 60°C / 95% relative humidity.
Operating conditions: 5 - 35°C / 95% relative humidity.
WEIGHT AND VOLUME (Packaged)
Weight: 24 kg.
Volume: 0.163 m³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
ALTERNATIVE PRODUCTS
S0002048, CPAP, bubble,medical,new-born
S0004057, CPAP,bubble,newborn,wcompressor & SpO2
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance – Collateral standard: Electromagnetic compatibility Requirements and tests
- ISO 80601-2-69:2014 - Medical electrical equipment -- part 2-69 : particular requirements for basic safety and essential performance of oxygen concentrator equipment
O2 and air connections compliant with German Standard, ISO 9170-1, DIN13620-2 and CE listed.
Related Products