S0004057
CPAP,bubble,newborn,w/compressor & SpO2
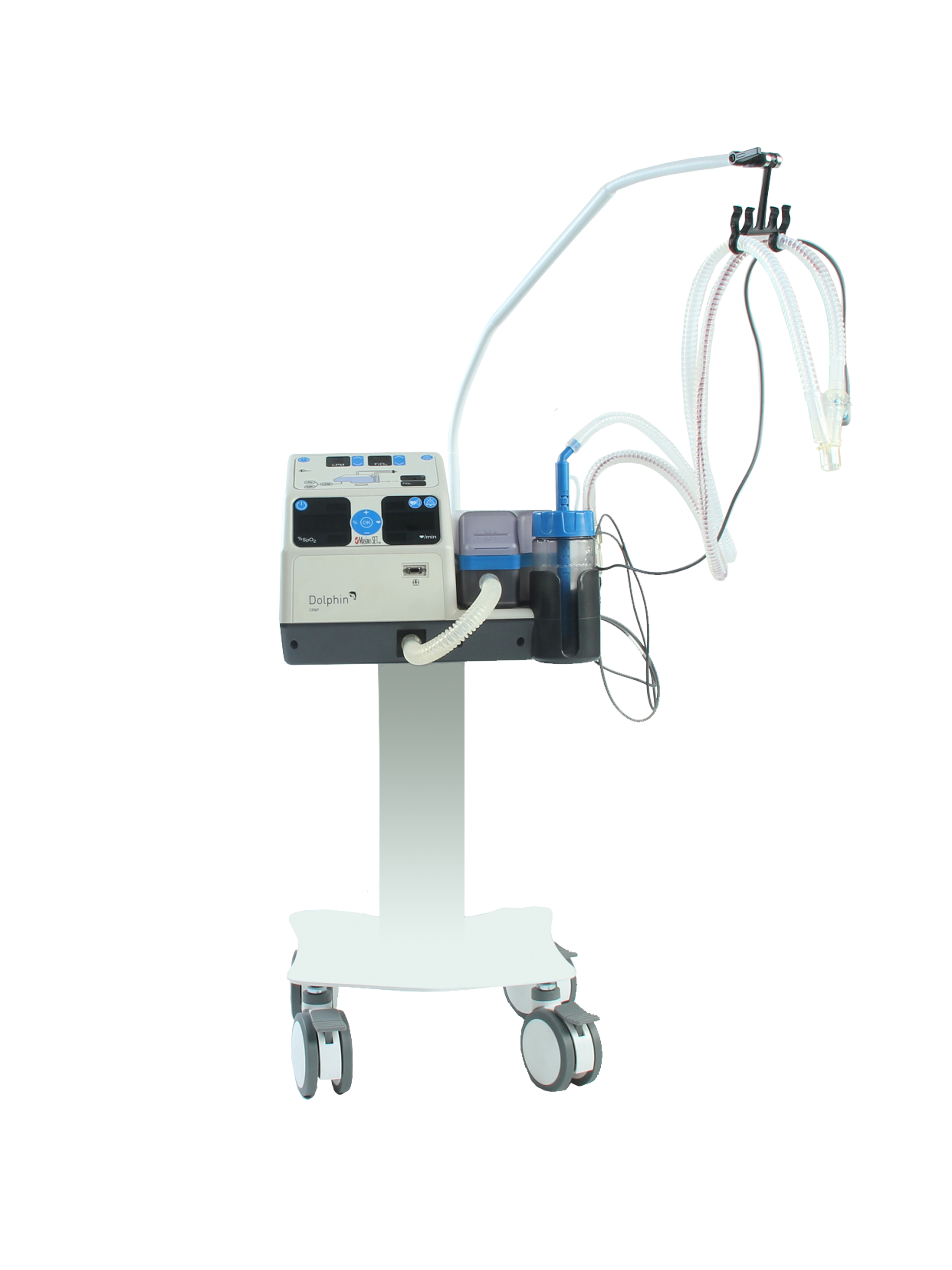
Bubble CPAP non-invasive respiratory support system, for all normal , premature and low birth-weight neonates. Comes with integrated air compressor, SpO₂, humidifier, air/oxygen mixer , and accessories.
Indicative Price 6,100.00 USD
GENERAL DESCRIPTION
Continuous Positive Airway Pressure (Bubble CPAP) system for the non-invasive respiratory support of normal, premature or low-birth weight neonate with spontaneous breathing. The unit has an integrated pulseoximeter to measure the level of arterial oxygen saturation in blood.
IMPORTANT
Training and installation are not included with the device and must be requested separately from the supplier or manufacturer. Improper operation or maintenance can result in harm to patients and/or operators, including death. Therefore, UNICEF strongly recommends the inclusion of installation and training services for both operators and technicians, unless identical models of these devices are already in use at the intended installation site. Neither UNICEF nor the manufacturer can assume responsibility for any consequences arising from incorrect operation and/or maintenance. Training and installation will have to be quoted separately; the number of operators and technicians to be trained, and the locations where these trainings will have to take place, are required in order to obtain a quotation.
INTENDED USE
An electrically-powered device designed to deliver high-flow (exceeding peak inspiratory flow) heated and humidified ambient air or air/oxygen to a neonatal patient as part of noninvasive ventilation (NIV); it may additionally be used with a water tank to produce bubble continuous positive airway pressure (bubble CPAP). It consists of a gas flow generator that entrains room air, an oxygen input, a heating element, and humidification chamber; it does not include CPAP controlling pressure sensors (i.e., not a full CPAP unit). It is intended for use by a healthcare provider on a spontaneously breathing patient in hospital settings.
TECHNICAL SPECIFICATIONS
The unit supports bubble CPAP Mode.
Specifically for support of neonates and newborns.
The unit has an integrated air compressor.
The pressure/flow of the CPAP generator is regulated electronically.
The unit has an integrated humidifier.
The unit is equipped with an electronic air/oxygen mixer.
The unit has an integrated FiO₂ analyser.
The unit accepts inlet gas supply pressures between 1.7 – 4.1 bar (25 to 60 psi).
DISS O₂ connection. (Other type connectors available indicate when ordering).
Supplied with pole mounting system, wheeled and with brakes.
The pole mounting system is equipped with 4 antistatic swivel castors, of which two castors. have been equipped with brakes.
Equipped with an air filter and a water trap.
Suitable for heated and non-heated closed patient circuits.
The unit accepts other than the manufacturer patient circuits.
All components of the system (single-use filters and patient circuits excluded) are suitable for disinfection with hospital grade products.
CPAP pressure range adjustable from 0 to 10 cm H2O.
Maintains constant CPAP at outlet.
A minimum output flow range adjustable between: 4 – 9 L/min.
Oxygen concentration adjustable between: 21 - 100 %.
Pressure indicator in cmH2O.
Noise emission less than 50 dB.
Including a clamp for a rail and/or pole.
External electric heating and humidifying chamber.
Relative humidity output up to 100%.
Adjustable output temperature up to 36°C.
Power requirements: 100 - 240 Volts - 50/60 Hz (not necessarily in a single unit).
ALARMS
Alarms are audible as well as visual.
The unit has alarms for the following:
- High temperature.
- System failure.
- High/low oxygen concentration (FiO₂).
- High airway pressure.
- Failure in either air or oxygen supply.
- Mains power failure.
- Low battery.
SAFETY PROVISIONS
Overheating protection.
The unit is equipped with a rechargeable battery which provides for a minimum of 1 hour autonomous operation.
In case of power failure the unit switches automatically to battery power.
The unit automatic recharges on reconnection to mains.
INTEGRATED PULSEOXIMETER
The unit is equipped with an integrated pulseoximeter.
Saturation accuracy between 70% and 100% is ±3%.
Measurement range SpO₂: 1% to 100% saturation .
Perfusion index: 0.02% to 20%.
Pulse rate range: 25 to 240 bpm.
Pluse rate accuracy is ±5%.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English.
1 x Plastic protective dustcover.
4 x reusable patient circuits.
2 sets of extra small size reusable CPAP head bonnets.
2 sets of small size reusable CPAP head bonnets.
2 sets of medium size reusable CPAP head bonnets.
10 x nasal cannulas, soft and atraumatic, for premature neonates.
10 x nasal cannulas, soft and atraumatic, for term neonates and older infants.
1 x set of hoses for connecting external oxygen and medical air supply, length > 2 m.
ESTIMATED LIFE SPAN
7 years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 19°C - 37°C / 30% – 90% RH.
- Storage conditions: 10°C - 65°C / 5% – 90% RH.
- Atmospheric pressure: 700 ~ 1060hPa.
- Ingress protection rating: IPX1.
WEIGHT AND VOLUME (packaged).
Weight: 28 kg.
Volume: 202 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS:
S0002031 Monitor,patient,portable,w/access.
S0002033 Pulse oximeter,portable,w/access.
ALTERNATIVE PRODUCTS
S0004002, CPAP,newborn,w/compressor & O₂ concentr.
S0002048, CPAP,bubble,medical,new-born
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-1-6:2010 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability.
- IEC 60601-1-8:2007 Medical electrical equipment — Part 1-8: General requirements for basic safety and essential performance — Collateral standard: General requirements, tests and guidance for alarm systems in medical electrical equipment and medical electrical systems.
- ISO 8185:2007 Respiratory tract humidifiers for medical use -- Particular requirements for respiratory humidification systems.
- ISO 80601-2-61:2011: Medical electrical equipment — Part 2-61: Particular requirements for basic safety and essential performance of pulse oximeter equipment.
NOMENCLATURE
- GMDN code: Neonatal high-flow respiratory unit (65490).
- UMDNS code: Positive Airway Pressure Units, Continuous (11001).
Related Products