S0004169
Ablation device,thermal,handheld,model 1
Portable Thermal Ablation Device for treating precancerous cervical lesions in women using a heated probe tip to destroy cervical tissue with abnormal cell growth.
Indicative Price 995.40 USD
GENERAL DESCRIPTION
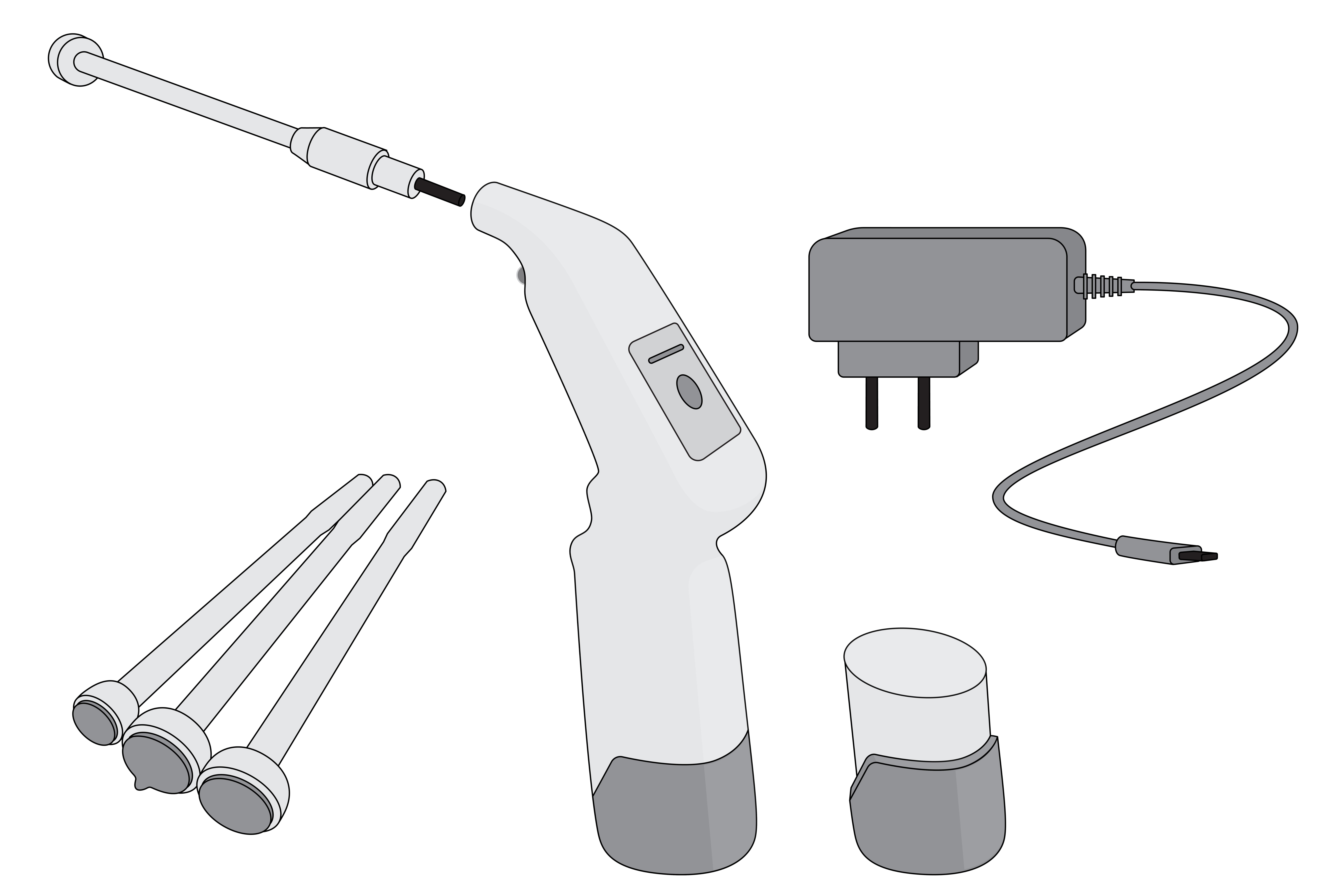
Thermal ablation device (thermocoagulator), battery operated, including 4 probes.
MANUFACTURER / MODEL
Manufacturer: Liger Medical LLC.
Country of manufacture: United States of America
Model name: Thermocoagulator.
Model reference: HTU-110.
Notes on pricing:
- Staircase pricing applies based on order volume.
- Unit price includes a 2.5% handling fee charged by the supplier for export order processing.
MOQ 1 piece.
INTENDED USE
A thermal ablation device is a self-contained, electrically powered medical instrument designed to destroy tissue of the uterine cervix with low-grade heat. This portable device uses a reusable metallic probe which is electrically heated to approximately 100° Celsius and applied for a period of 20 – 30 seconds to the affected area. This will result in epithelial and stromal destruction of the lesion. It may also be referred to as thermal coagulation or Semm cold coagulation, after the inventor of the device. The term “cold” has been used due to a treatment temperature of 100°C, which is lower than that used for standard clinical electrocautery (usually between 400-600°C).
TECHNICAL SPECIFICATIONS
Probe tip temperature is controlled to reach 100°C, users can increase up to 120°C.
Visual and audible cues provide feedback to user to ensure working temperature reached.
An automatic timer shuts down the heating after 20 seconds.
Device is simple and easy to use, appropriate for all levels of care.
Device features built-in full spectrum light source for viewing and assessing the cervix.
The device is portable and lightweight, easily carried in a case or backpack, together with all its accessories.
The device comes either with a pouch or a plastic case for storage purposes and to protect the device during transport.
Included 4 probes:
- 2 x flat probe 19 mm.
- 1 x flat probe 16 mm.
- 1 x conical probe, with a nipple extrusion, 19 mm (allows to anchor in center of cervix but not to ablate endocervix).
Probes do not have sharp edges, are made from biocompatible material that will not adhere to cervix and are removable and reusable.
The shaft and tips can be decontaminated between uses by High Level Disinfection or autoclaving. Consult user instructions for details on decontamination procedures.
Probe shaft should itself remain at safe low temperatures to prevent burns to the vaginal walls.
The unit cannot be operated directly from the mains power supply, only from the included batteries.
Fully charged battery have a cumulative run-time of at least 1 hour or are capable to treat a minimum of 30 patients on a single charge.
The batteries are rechargeable. Units come with a charger, suitable for a mains power supply in the range of: 110-220 V, 50-60 Hz power.
SUPPLIED WITH
Instructions for use in English, French, Spanish and Portuguese.
2 x probe flat 19 mm
1 x flat probe 16 mm.
OPTIONAL ITEMS ADDED AT COST
Additional probes:
- 16 mm flat
- 19 mm flat
- 19 mm conical with nipple
Additional batteries
LIFE SPAN
Between 5 years for the device (probes life span approximately 120 cycles).
WARRANTY
Two years warranty after shipment excluding the battery.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 16°C - 45°C / <80 % RH
- Storage conditions: -5°C - 45°C / <80 % RH
WEIGHT AND VOLUME (packaged)
Weight: 1.8 kg
Volume: 5.168 dm3
ESTIMATED DELIVERY LEAD TIME
One week.
INSTALLATION REQUIREMENTS
There is no need for a qualified technician to assemble or commission this device.
TRAINING REQUIREMENTS
Should only be used by trained medical professionals.
MAINTENANCE/USER REQUIREMENTS
As per user manuals.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
CLASSIFICATION
CE certified as class IIa device under MDD 93/42/EEC.
SAFETY & PRODUCT STANDARDS
Compliant with active version of the following standards for General Manufacturing (or equivalent):
- ISO 14971: Medical Devices - Application of Risk Management to Medical Devices.
- ISO 15223-1: Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements.
- IEC 60601-1 - Medical electrical equipment - Part 1: General requirements for basic safety and essential performance. Please provide the relevant certification or test report.
- IEC 60601-1-2: Medical electrical equipment - Part 1-2 General requirements for basic safety and essential performance - Collateral Standard: Electromagnetic disturbances - Requirements and tests. Please provide the relevant certification or test report.
BATTERY
- IEC 62133 - Secondary cells and batteries containing alkaline or other non-acid electrolytes – Safety requirements for portable sealed secondary cells:»Part 1: Nickel»Part 2: Lithium."
PROBES
- ISO 10993-1: Biological evaluation of medical devices Part 1: Evaluation and testing within a risk management process.
n a risk management process.
Related Products