S0004225
Consumables, CPAP,6mos,Diamedica,Hudson
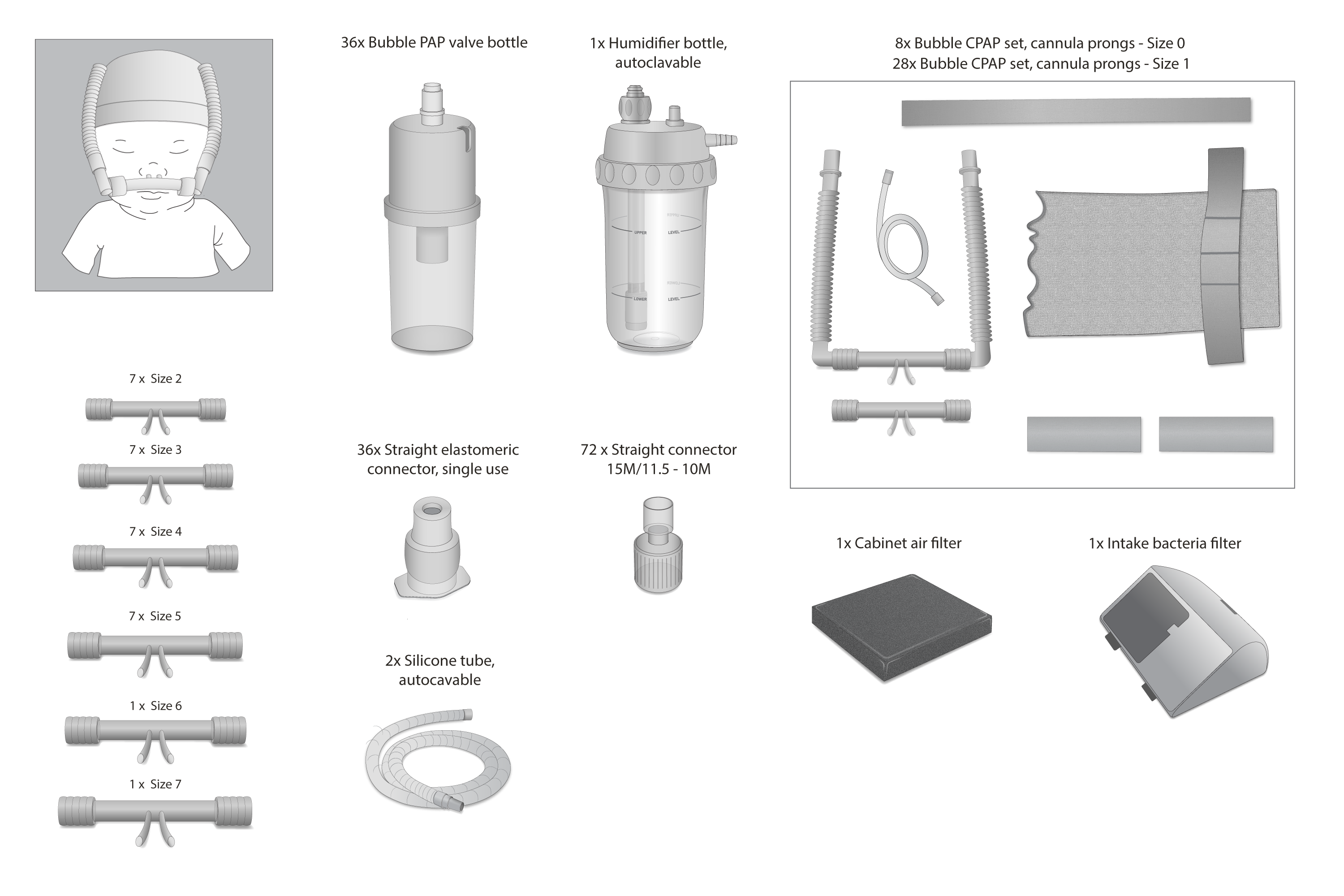
Consumables bundle for the Diamedica CPAP 20. Bundle is for one CPAP device for an estimated 6 months of use, assuming a 5-day patient stay. This bundle contains Hudson-style nasal cannulas to support neonatal CPAP patients with weights ranging from less than 700 grams to greater than 3000 grams.
Indicative Price 2,991.65 USD
GENERAL DESCRIPTION
Consumables bundle for the Diamedica CPAP 20. Bundle is for one CPAP device for an estimated 6 months of use, assuming a 5-day patient stay. This bundle contains Hudson-style nasal cannulas to support neonatal CPAP patients with weights ranging from less than 700 grams to greater than 3000 grams.
TECHNICAL SPECIFICATIONS
8 x Bubble CPAP set, silicone cannula prongs – Size 0 (016-001-000)
28 x Bubble CPAP set, silicone cannula prongs – Size 1 (016-001-001)
7 x Silicone prong – Size 2 (016-012-002)
7 x Silicone prong – Size 3 (016-012-003)
7 x Silicone prong – Size 4 (016-012-004)
7 x Silicone prong – Size 5 (016-012-005)
1 x Silicone prong – Size 6 (016-012-006)
1 x Silicone prong – Size 7 (016-012-007)
2 x Silicone tube, autoclavable (002-002-3150E)
72 x Straight connector 15M/11.5 – 10M, single use (1661000)
36 x Straight elastomeric connector, single use (1702000)
36 x Bubble PAP valve bottle, single use (AN0018)
1 x Humidifier bottle, autoclavable (H110)
1 x Intake bacteria filter (1025D-605)
1 x Cabinet air filter (303DZ-605)
STORAGE CONDITIONS
Temperature: store within -10 to 40°C (range varies per component)
Relative humidity: keep dry
WARRANTY
Includes 2-year standard warranty, dated from the completion of the terms of delivery.
SHELF LIFE
5 years (from date of manufacture of individual components)
WEIGHT/VOLUME
2 cartons
Total weight: 14.5kg (6.5kg + 8kg)
Volume (m³): 0.152m³ (0.066m³ + 0.086m³)
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices – Quality management systems – Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
REGULATION & CONFORMITY REQUIREMENTS
Medical consumables have CE certificate under EU MDD 93/42/ECC or EU MDR 2017/745.
Related Products