S0145558
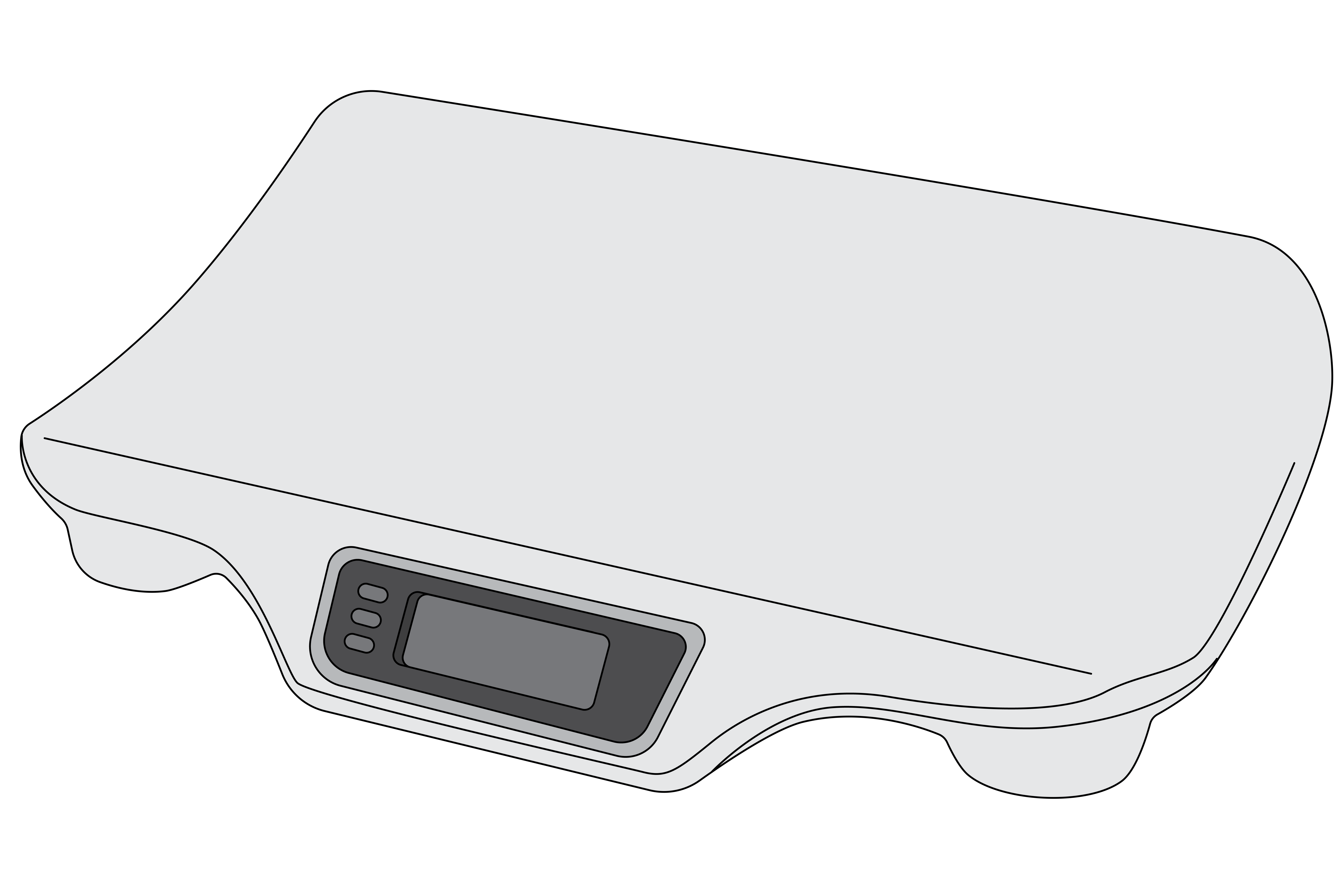
Scale,infant,tray,20kgx5g,batteries
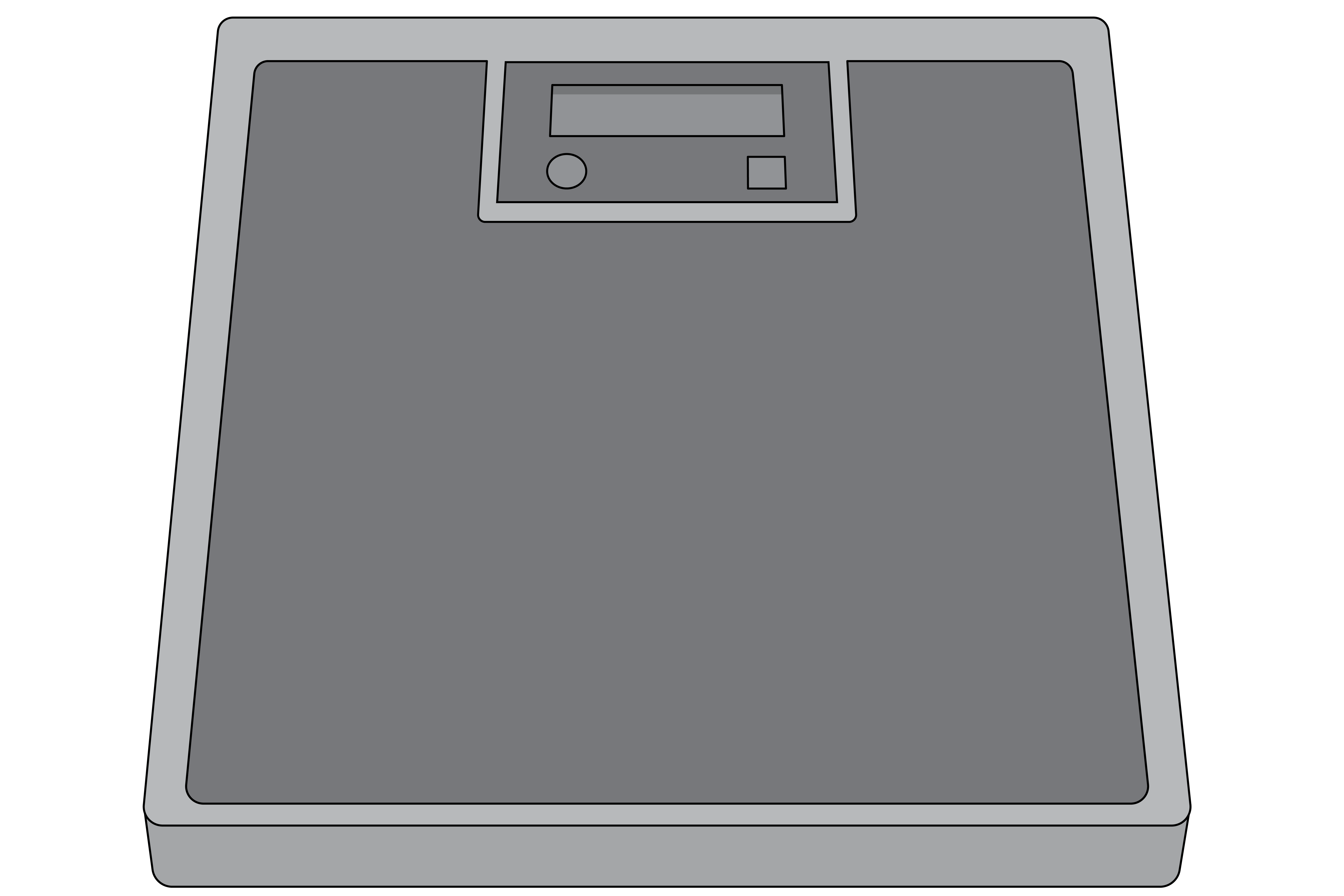
Electronic scale for weighing infants up to 20 kg.
Indicative Price 131.85 USD
General Description:
Scale, infant, tray, 20kg x 5g ,batteries
Intended use:
Electric measuring device to weigh newborns or monitor weight changes for infants in health facilities or hospitals. Light-weight and portable for home visits.
Technical Specifications:
Weighing range: up to 20 kg.
Minimum graduation: 5 g.
Accuracy: ±5 g.
Precision: ±5 g.
Display: kg.
Tare function.
Autohold function.
Automatic switch-off.
Auto-calibration with each switch-on.
Large LCD display.
Reading time max 5 seconds.
Splash proof and shock resistant.
Smooth surface/finishing for easy cleaning/disinfection.
Material: Body and tray made of non absorbent ABS plastic.
Colour: white or light grey.
With or without levelling feet.
Dimensions:
Baby tray: (525-600) x (40-80) x (250-280) mm (WxHxD)
Overall: (525-600) x (130-156) x (332x385) mm (WxHxD)
Weight: 2.3-2.85 kg
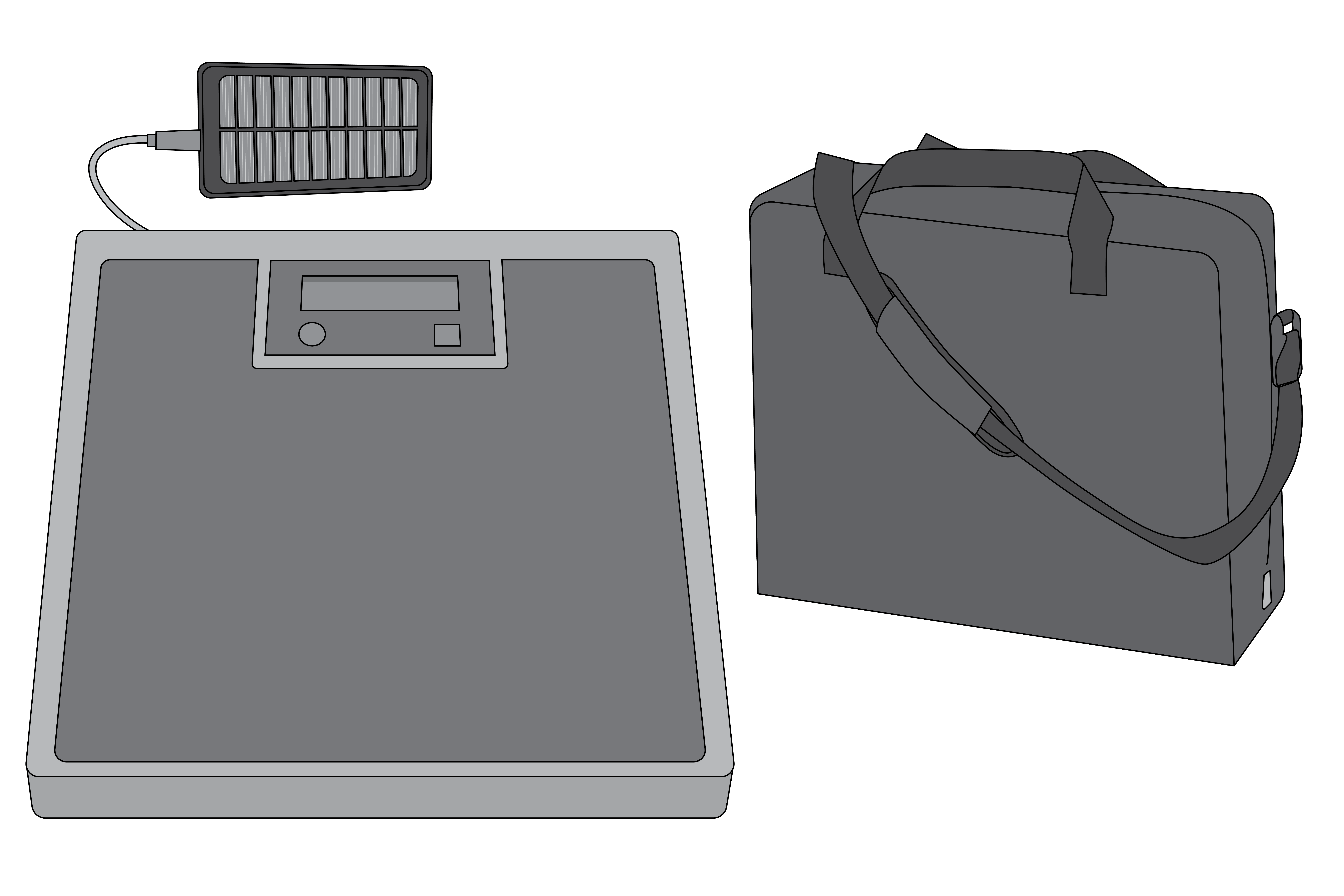
Power sources:
Can be powered by battery power or power adapter:
Batteries: 4 AA batteries (customer replaceable or rechargeable).
Adapter: 100 - 240 V / 50-60 Hz, 0.2 Amp.
Environmental conditions:
Operating temperature: minimum range: 0 to 45 degrees C.
Storage/transport temperature: minimum range: -20 to 65 degrees C.
Humidity: up to 80% RH.
Supplied with:
Instructions for assembly, use, and cleaning in English, French and Spanish; illustrated with pictograms.
Separately packed batteries.
AC adapter with multi-country plugs.
Contact details for repair service and contact details for recalibration services.
Warranty and service:
Warranty: 2 or 3 years.
Shelf life: None or 5 years
Product lifespan: 7 or 8 years.
After sales service available for recalibration and repair in different parts of the world.
Quality Management System:
ISO 13485:2016 Medical devices - Quality management
Regulation & classification:
Medical Device Regulation (EU) 2017/745, Class Im or
Medical Device Directive 93/42/EEC, Class Im
Compliance to safety & product performance standards:
EN 60601-1: Medical electrical equipment - General requirements for basic safety and essential performance.
IEC 60601-1-2: Electromagnetic Compatibility of Medical Devices or EN 60601-1-2: Electromagnetic disturbances - Requirements and tests.
Nomenclature:
GMDN code: 35324 - Infant scale, electronic
Primary packaging:
One (1) unit per box
Related Products