S0683200
Sphygmomanometer,(adult),aneroid
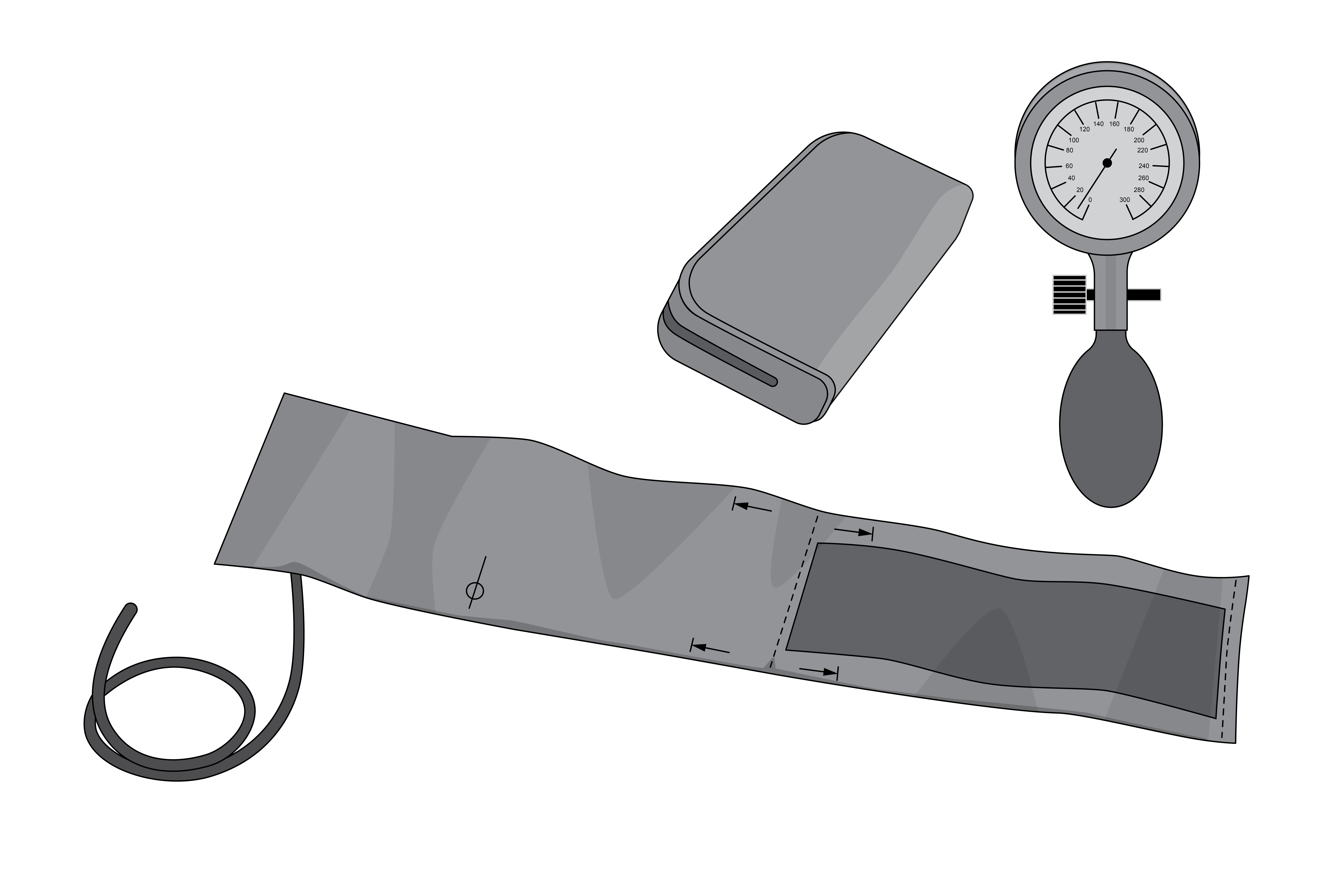
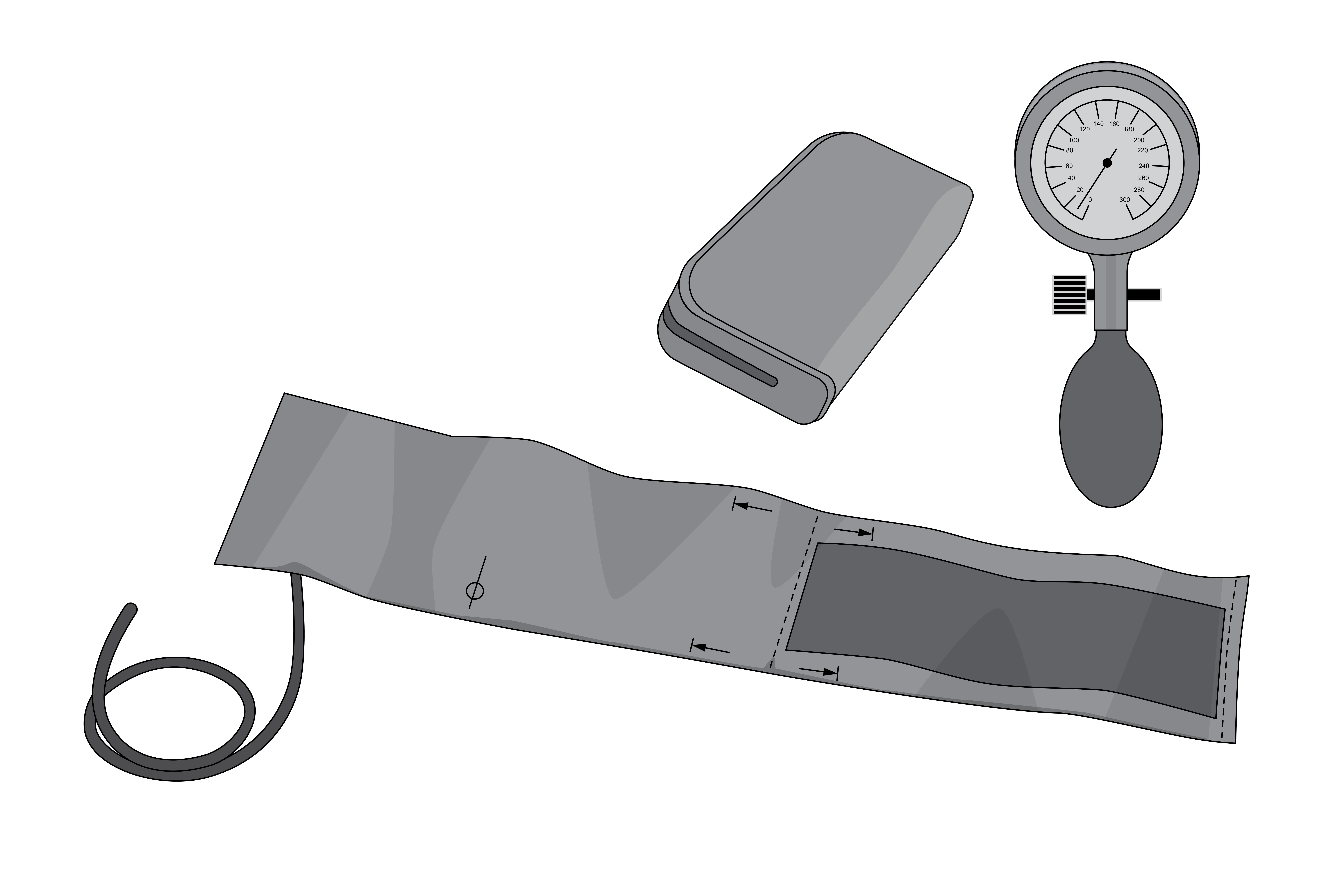
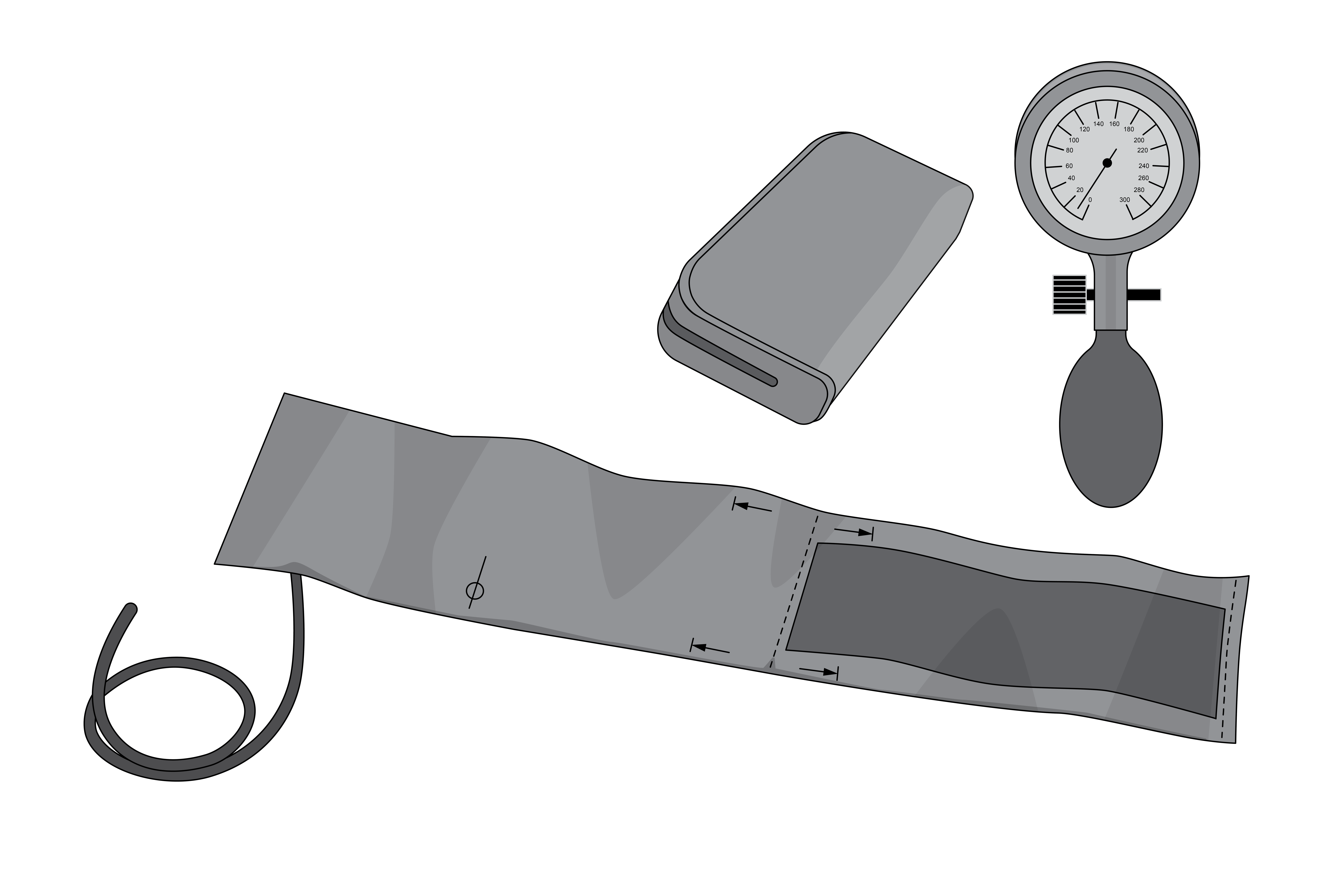
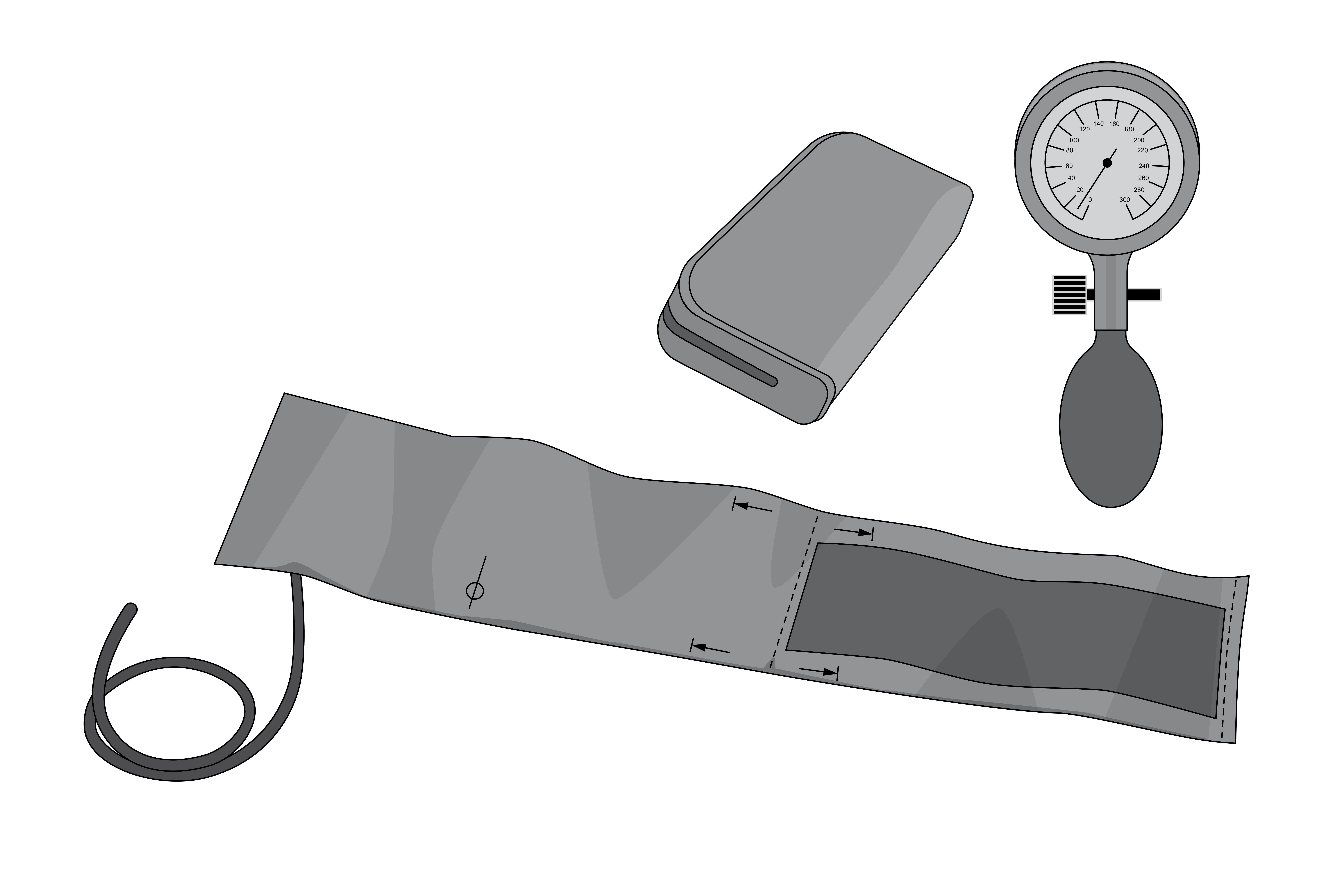
Sphygmomanometer (adult), aneroid sphygmomanometer. Measures blood pressure non-invasively by displaying the pressure in a cuff wrapped around a patient’s arm. The systolic and diastolic pressure is usually assessed by listening to Korotkoff sounds generated by arterial blood flow using a stethoscope simultaneously.
Indicative Price 12.20 USD
GENERAL DESCRIPTION
Sphygmomanometer,(adult),aneroid
INTENDED USE
A device designed to measure blood pressure consisting of an inflatable cuff that fits around a limb (arm or thigh), an inflation bulb for controlling the air pressure within the cuff, an aneroid manometer, and tubing. The aneroid manometer consists of a metal bellows, which expands as the pressure in the cuff increases, and a mechanical amplifier that transmits this expansion through a lever to an indicator needle, which rotates around a circular, calibrated scale. The manometer is hand held (portable); blood pressure measurement is taken in conjunction with a stethoscope.
TECHNICAL SPECIFICATIONS
Composed of a cuff containing an inflatable bag.
The inflatable bag is connected via a tube to a bulb with an integrated manometer needle gauge.
The cuff is made of durable material (e.g. nylon), which is non-deformable, and washable at 30°C.
The cuff is fitted with double Velcro fastening, enabling a tight and secure fit around arms.
The cuff is reinforced at both sides.
Size cuff for adult.
The bag is inflated by means of the flexible bulb connected via a tube.
Material tube: rubber or other suitable material, e.g. silicone rubber, crack resistant.
Length tube between: 50 to 70 cm.
Gauge graduated 0 - 300mmHg (min) in 2 (max) mmHg increments, with pressure release valve.
Accuracy as per ISO 81060-1: +/- 3mm Hg.
Latex and mercury free design.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x Plastic protective case or pouch.
SHELF LIFE
Not applicable.
ESTIMATED LIFE SPAN
Five to ten years depending on the model supplied.
WARRANTY
Two years.
ENVIRONMENTAL CONDITIONS
Storage conditions: -20 - 70°C / 85% RH.
Operating conditions: 10 - 40°C / 85% RH.
WEIGHT AND VOLUME (Packaged)
Weight: 400 gr.
Volume: 1.26 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
There are no installation or commissioning requirements.
TRAINING REQUIREMENTS
Where staff has experience with these types of devices, no user training will be required.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements.
RELATED PRODUCTS
S0683300, Sphygmomanometer,(child),aneroid
S0845188, Sphygmomanometer,(child),resilient
ALTERNATIVE PRODUCTS
S0845187, Sphygmomanometer,(adult),resilient
COMPONENT OF A KIT
S9901029 - IEHK2017,kit,suppl.2-equipment
S9903002 - AWD, Periphery kit, Equipment
S9902221 - Midwifery kit – equipment, 2
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under EU MDD 93/42 as a Class Im device, or CE certified under EU MDR 2017/745 as Class Im device.
SAFETY AND PRODUCT STANDARDS
- ISO 81060-1 Non-invasive sphygmomanometers - Part 1: Requirements and test methods for non-automated measurement type.
- ISO 10993-1, Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing within a Risk Management Process.
- EN ISO 15223-1 (EN 980) Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements.
NOMENCLATURE
GMDN Code: 16156
Related Products