S0845187
Sphygmomanometer,(adult),resilient
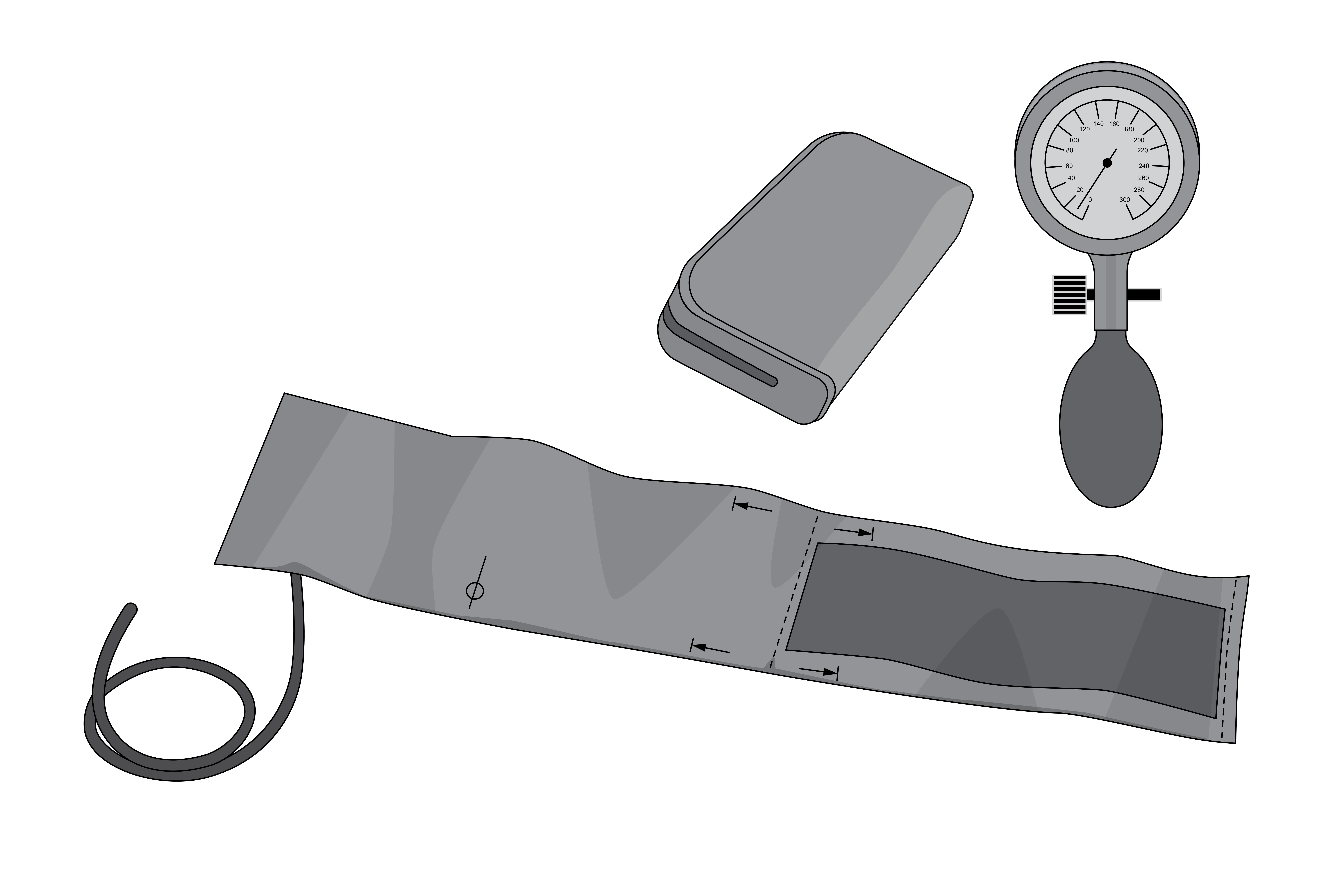
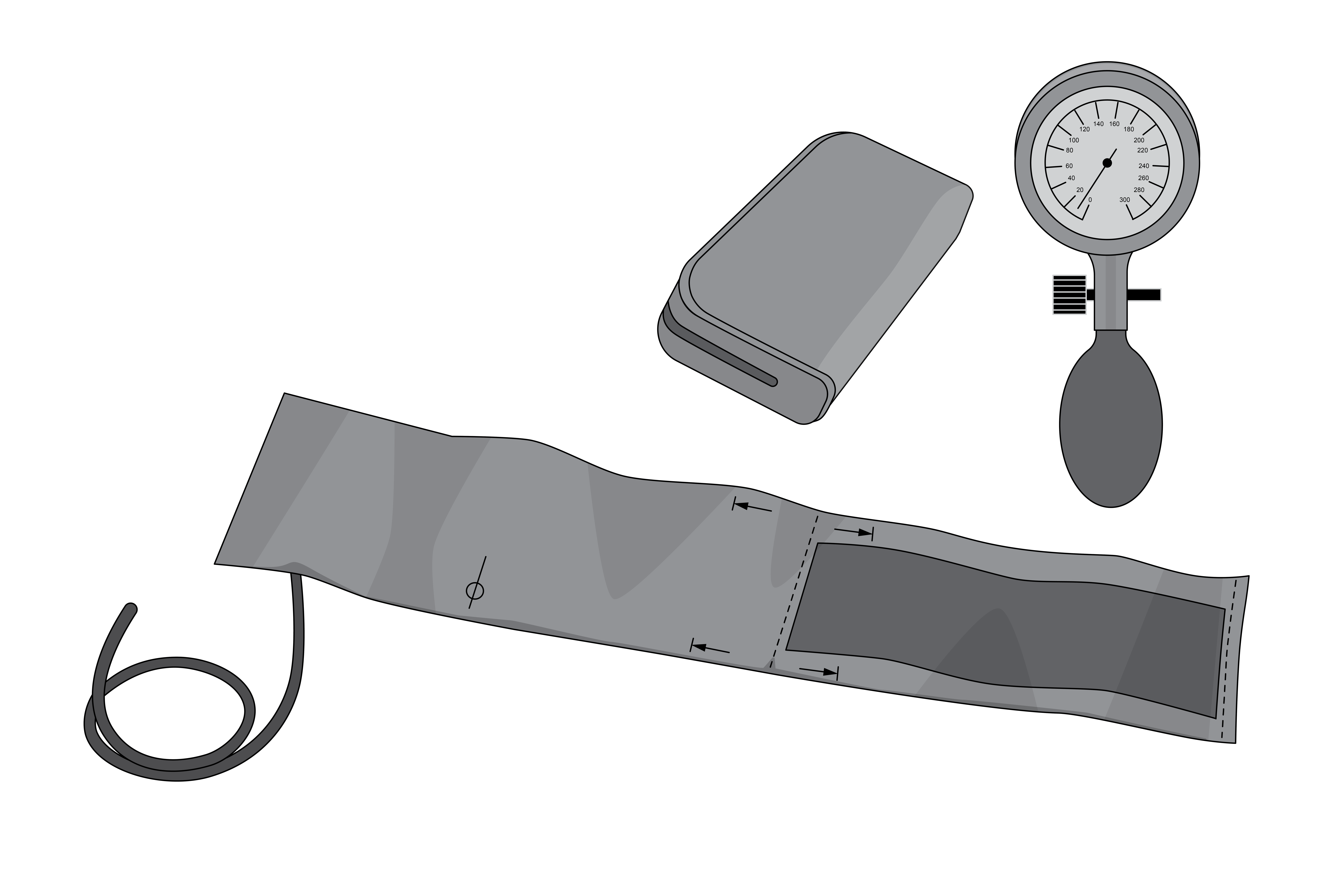
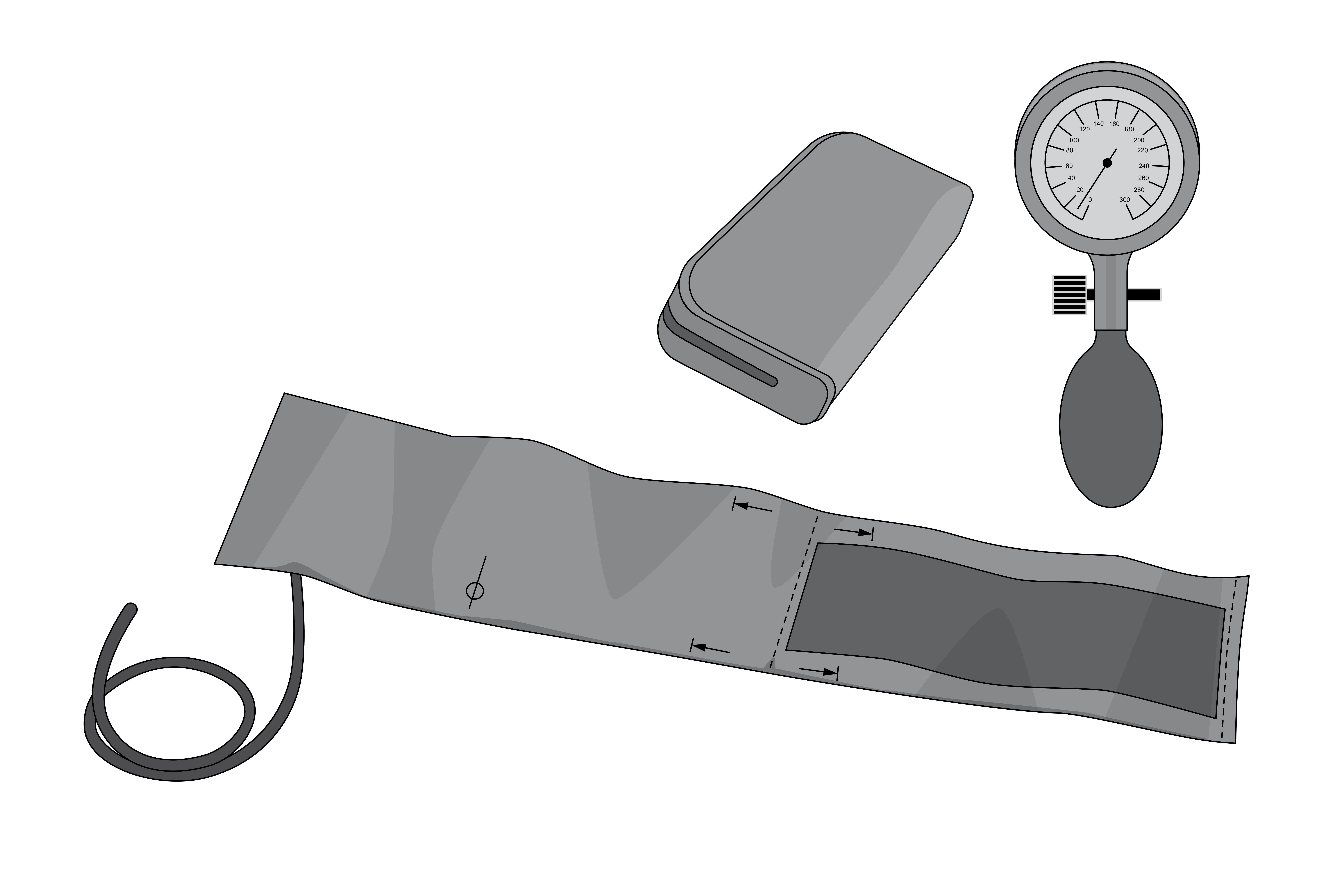
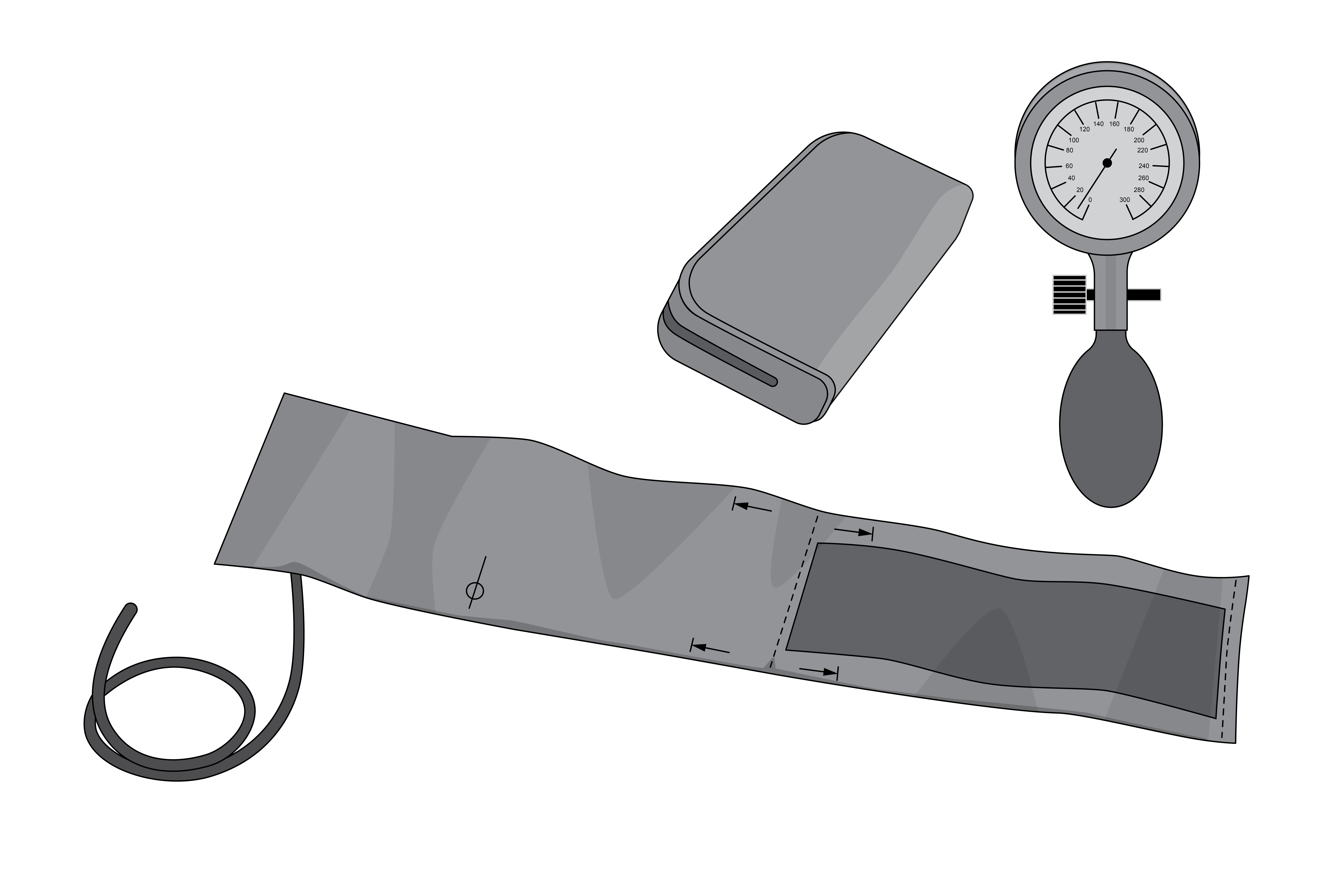
Sphygmomanometer (adult), aneroid sphygmomanometer. Measures blood pressure non-invasively by displaying the pressure in a cuff wrapped around a patient’s arm. The systolic and diastolic pressure is usually assessed by listening to Korotkoff sounds generated by arterial blood flow using a stethoscope simultaneously.
Indicative Price 35.19 USD
GENERAL DESCRIPTION
Sphygmomanometer,(adult),aneroid
INTENDED USE
A device designed to measure blood pressure consisting of an inflatable cuff that fits around a limb (arm or thigh), an inflation bulb for controlling the air pressure within the cuff, an aneroid manometer, and tubing. The aneroid manometer consists of a metal bellows, which expands as the pressure in the cuff increases, and a mechanical amplifier that transmits this expansion through a lever to an indicator needle, which rotates around a circular, calibrated scale. The manometer is hand held (portable); blood pressure measurement is taken in conjunction with a stethoscope.
TECHNICAL SPECIFICATIONS
Composed of a cuff containing an inflatable bag.
The inflatable bag is connected via a tube to a bulb with an integrated manometer needle gauge.
The cuff is made of durable material (e.g. nylon), which is non-deformable, and washable at 30°C.
The cuff is fitted with double Velcro fastening, enabling a tight and secure fit around arms.
The cuff is reinforced at both sides.
Size cuff for adult.
The bag is inflated by means of the flexible bulb connected via a tube.
Material tube: rubber or other suitable material, e.g. silicone rubber, crack resistant.
Length tube: 70 cm.
Equipped with a universal quick connector for connection inflation bulb.
Gauge graduated 0 - 300mmHg (min) in 2 (max) mmHg increments, with pressure release valve.
Accuracy as per ISO 81060-1: +/- 3mm Hg.
Latex and mercury free design.
This model is designed to withstand shocks and vibration.
Gauge body to allow recalibration of readings, yet in normal operation be sealed and secure. Calibration should only be done by the manufacturer or a authorized dealer.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x Plastic protective case or pouch.
SHELF LIFE
Not applicable.
ESTIMATED LIFE SPAN
Ten years.
WARRANTY
Two years.
ENVIRONMENTAL CONDITIONS
Storage conditions: -20 - 70°C / 85% RH.
Operating conditions: 10 - 50°C / 85% RH.
WEIGHT AND VOLUME (Packaged)
Weight: 400 gr.
Volume: 1.23 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
There are no installation or commissioning requirements.
TRAINING REQUIREMENTS
Where staff has experience with these types of devices, no user training will be required.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements.
RELATED PRODUCTS
S0683300, Sphygmomanometer,(child),aneroid
S0845188, Sphygmomanometer,(child),resilient
ALTERNATIVE PRODUCTS
S0683200, Sphygmomanometer,(adult),aneroid
COMPONENT OF A KIT
No.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under EU MDD 93/42 as a Class Im device.
SAFETY AND PRODUCT STANDARDS
- ISO 81060-1 Non-invasive sphygmomanometers - Part 1: Requirements and test methods for non-automated measurement type.
- ISO 10993-1, Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing within a Risk Management Process.
- EN ISO 15223-1 (EN 980) Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements.
NOMENCLATURE
GMDN Code: 16156
Related Products