S0791500
Vacuum extractor,Bird,manual,comple.set
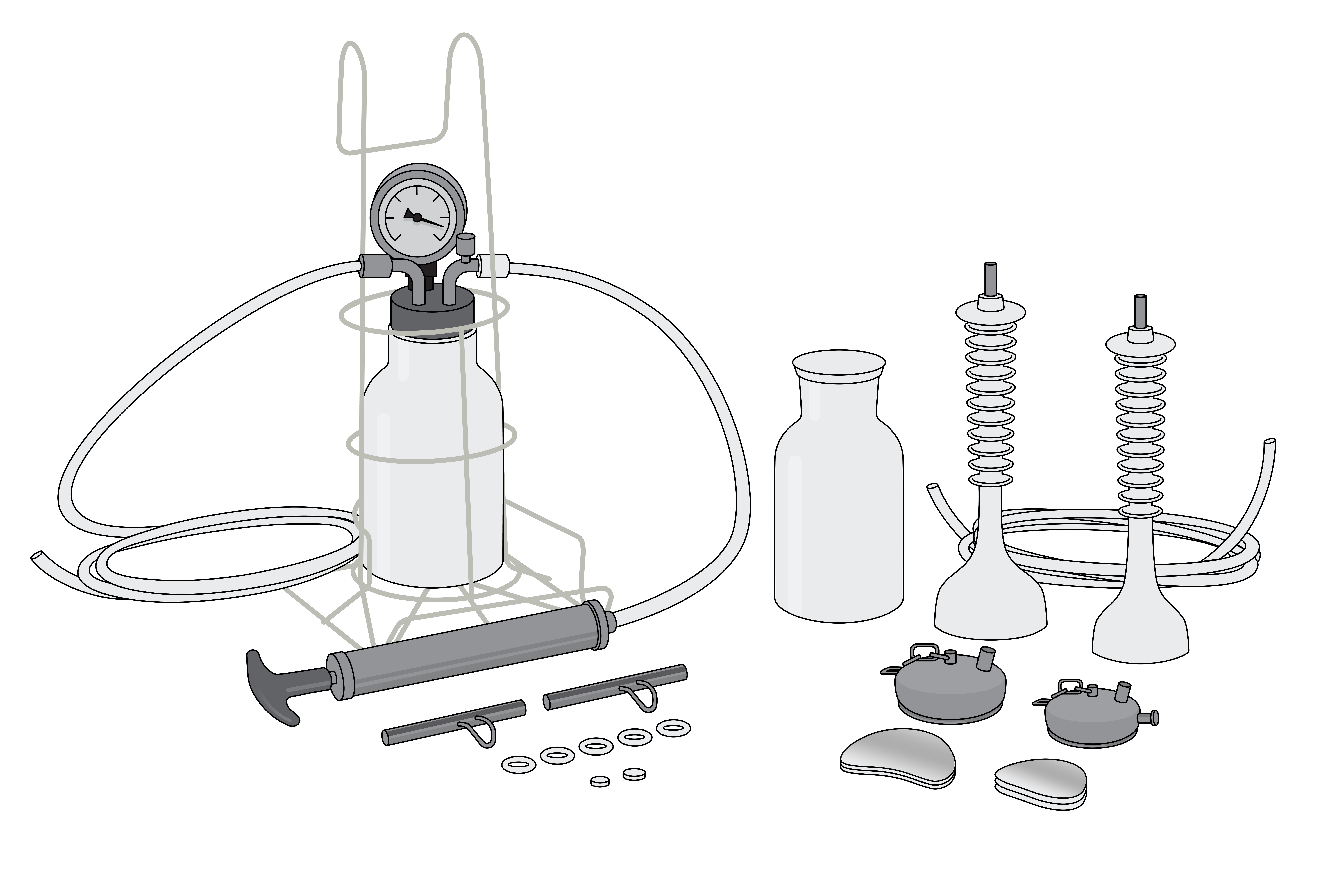
Vacuum extractor, Bird (anterior), manual, including two sizes Bird type anterior, two sizes soft cups, and all other required accessories.
Indicative Price 524.00 USD
GENERAL DESCRIPTION
Vacuum extractor, Bird (anterior), manual, complete set.
INTENDED USE
An assembly of hand-operated devices designed to facilitate the delivery of a foetus during vaginal childbirth or Caesarean section by enabling traction to be applied to the foetal head by means of a suction cup that is attached to the scalp. It typically consists of a cup with a soft inner liner to prevent tissue damage, a hand-operated pump, and a handle used to apply traction. It is typically used for dystocia, uterine inertia, maternal exhaustion, maternal/foetal distress. This is a reusable device.
TECHNICAL SPECIFICATIONS
Vacuum extractor system to assist vaginal deliveries.
Hand-operated suction pump supports the vacuum extraction.
Vacuum range, adjustable: from 0 to -85kPa/-638mmHg
Gauge dial, minimal graduation: 5kPa/40mmHg
Accuracy vacuum gauge: ± 2.5kPa
All parts of the vacuum extractor can be disassembled.
All parts can be disassembled and are autoclavable at 121°C
Requires no other than routine maintenance, manageable at the level of delivery rooms.
SYSTEM IS SUPPLIED AS A COMPLETE SET CONSITSTING OF
1 x Vacuum pump
2 x Bird type anterior suction cup, stainless steel: diameter 50 and 60mm rounded edges
2 x Soft vacuum extractor cups "silc-cup": diameter 50mm and 60mm
2 x Extraction handle, stainless steel
1 x Cover plug for vacuum collection bottle, with 3 holes (2 connectors +1 vacuum gauge)
1 x Connector, angled, chrome plated
1 x Connector angled with screw valve, chrome plated screw, cap and gasket
1 x Vacuum gauge (0 to -100 kPa/- 800mmHg) with straight connector, chrome plated
1 x Vacuum collection bottle, plastic 500ml
1 x Basket, epoxy coated, supporting vacuum collection bottle and the vacuum pump, with large hook allowing to fit it to the delivery table.
2 x Tube, silicone transparent, inner/outer diameter approx.: 6/11mm, respective lengths: 50 and 150cm
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x Spare vacuum bottle, plastic, 500ml.
1 x Spare tube, transparent silicone, inner/outer diameter: 6/11mm, length: 300cm.
1 x Spare gasket for the screw valve (vacuum release).
20 x Polypropylene bottom plate, for stainless steel suction/extraction cup, 10 for each size, 50 and 60mm.
1 x List of accessories and parts, each identified with their product reference.
ESTIMATED DELIVERY LEAD TIME
6 weeks
ESTIMATED LIFE SPAN
Guaranteed for 250 cycles without loss of performance.
WARRANTY
Two years.
ENVIRONMENTAL CONDITIONS
Storage conditions: -20 - 50°C / 20 - 95% RH.
Operating conditions: +5 - 40°C / 30 - 75% RH.
Atmospheric pressure: 70 to 106 kPa
WEIGHT AND VOLUME (Packaged)
Weight: 3.2 kg.
Volume: 18.0 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
This device does not have installation or commissioning requirements other than connecting tubing.
TRAINING REQUIREMENTS
Vacuum extraction should only be performed or supervised by trained and experience physicians. The manufacturer does not provide clinical training on vacuum extraction. The instructions for use included with the devices provides the necessary details for physicians trained on carrying out vacuum extractions not familiar with this specific device.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements.
ALTERNATIVE PRODUCTS
S0001000, Vacuum extractor,Bird(ant post),elec,set
S0791501, Vacuum extractor,Bird(ant&post),manu,set
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDD 93/42/EEC as Class IIa device.
SAFETY AND PRODUCT STANDARDS
- EN ISO 10079-2:2009 Medical suction equipment - Part 2: Manually powered suction equipment.
- ISO 10993-1:2018 Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process.
- ISO 10993-5:2009 Biological evaluation of medical devices. Tests for in vitro cytotoxicity.
NOMENCLATURE
GMDN Code: 32596, 63205, 42834, 16779.
Related Products