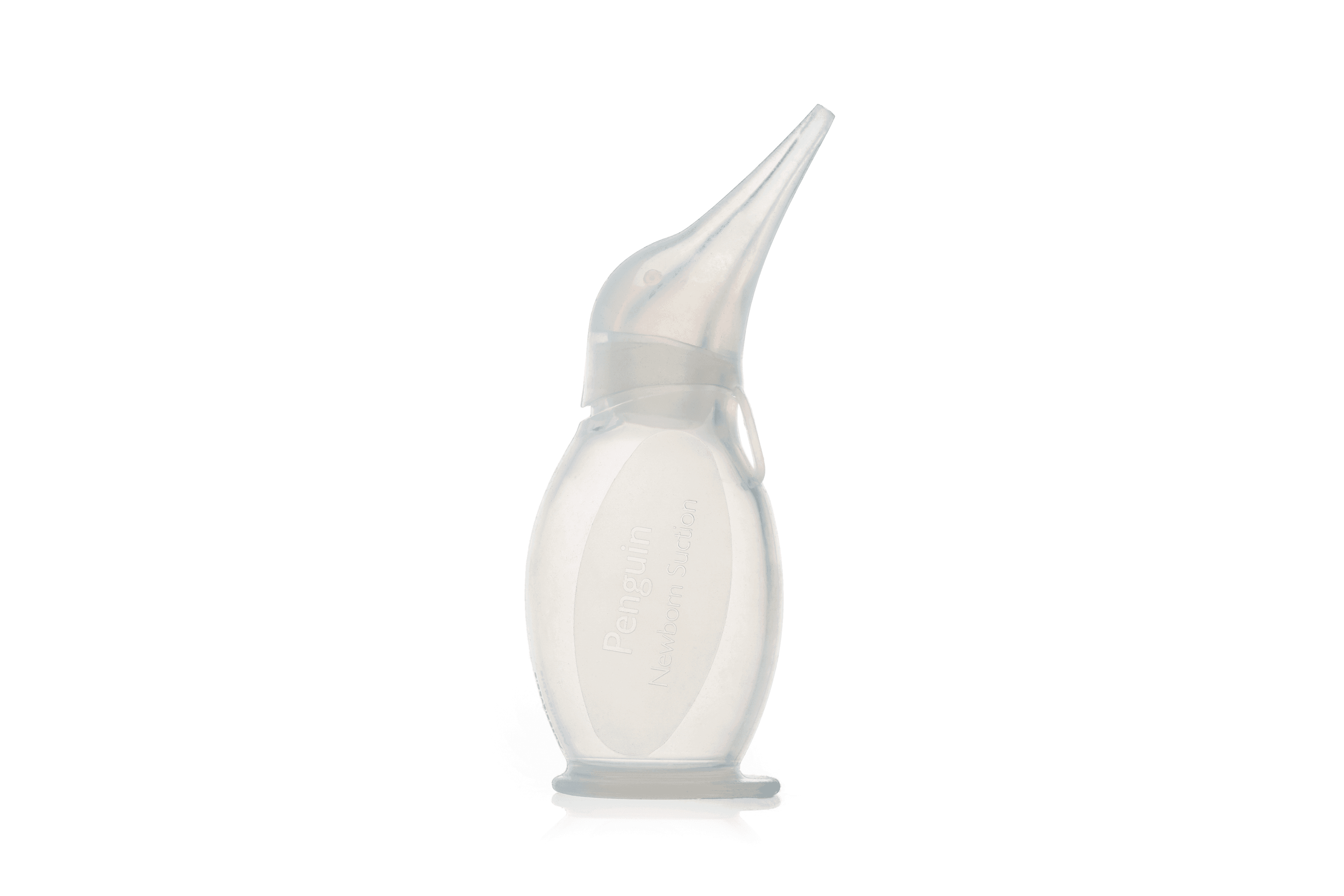S0845006
Bulb, suction, neonatal, reusable
A reusable, hand-held, manual suction device designed to suck gently and clear excessive mucus from the airway of a newborn to facilitate easier breathing. Can be diassembled for cleaning and sterilized for disinfection purposes.
Indicative Price 6.77 USD
GENERAL DESCRIPTION
Reusable, silicone bulb for manual suction of airways of newborns.
INTENDED USE
A reusable, hand-held, manual suction device designed to suck gently and clear excessive mucus from the airway of a newborn to facilitate easier breathing.
TECHNICAL SPECIFICATIONS
Reusable
Fully made from transparent or translucent material
Material: silicone or any material fulfilling ISO 10993-4:2002 or equivalent
No use of natural rubber latex
Nozzle dimensions at tip: Inner diameter (ID) 3.0mm ±5%
Nozzle dimensions at tip: Outer diameter (OD) 4.5mm ±5%
Suction strength should reach: 100mmHg (136cm H2O)
Fluid capacity: 75 ml
Can be autoclaved at 136°C and withstand other means of High Level Disinfection
Can be disassembled (opened up) for proper cleaning. After opening up it should be easy to access the internals of the bulb allowing for thorough cleaning.
ESTIMATED LIFE SPAN
The life span of the device is minimum 3 years or 20 autoclave sessions, whichever comes first.
WARRANTY
Two years from shipping date
ENVIRONMENTAL CONDITIONS
- Operating conditions: 5°C - 20°C / 90% RH
- Storage conditions: -20°C - 60°C / 90% RH
WEIGHT AND VOLUME
Weight: 0.11 kg
Volume: 0.55 dm³
ESTIMATED DELIVERY LEAD TIME
65 days
INSTALLATION REQUIREMENTS
The product does not require assembly or commissioning
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS
S0845009, Resuscitator,hand-oper.,child,set
S0845151, Resuscitator,hand-oper.,adult,set
S0845152, Resuscitator, upright, neonate, set
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class I device.
SAFETY & PRODUCT STANDARDS
En 1041 Information supplied by the manufacturer of medical devices
ISO 10993-1 Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process.
ISO 15223-1 Medical devices — Symbols to be used with information to be supplied by the manufacturer — Part 1: General requirements
EN 62366 Medical devices — Part 1: Application of usability engineering to medical devices
NOMENCLATURE
- GMDN code: Nasal aspirator, manual (41826)
- UMDNS code: Airway Obstruction Removal Devices, Emergency (10058)
Related Products