S0845151
Resuscitator,hand-oper.,child,set
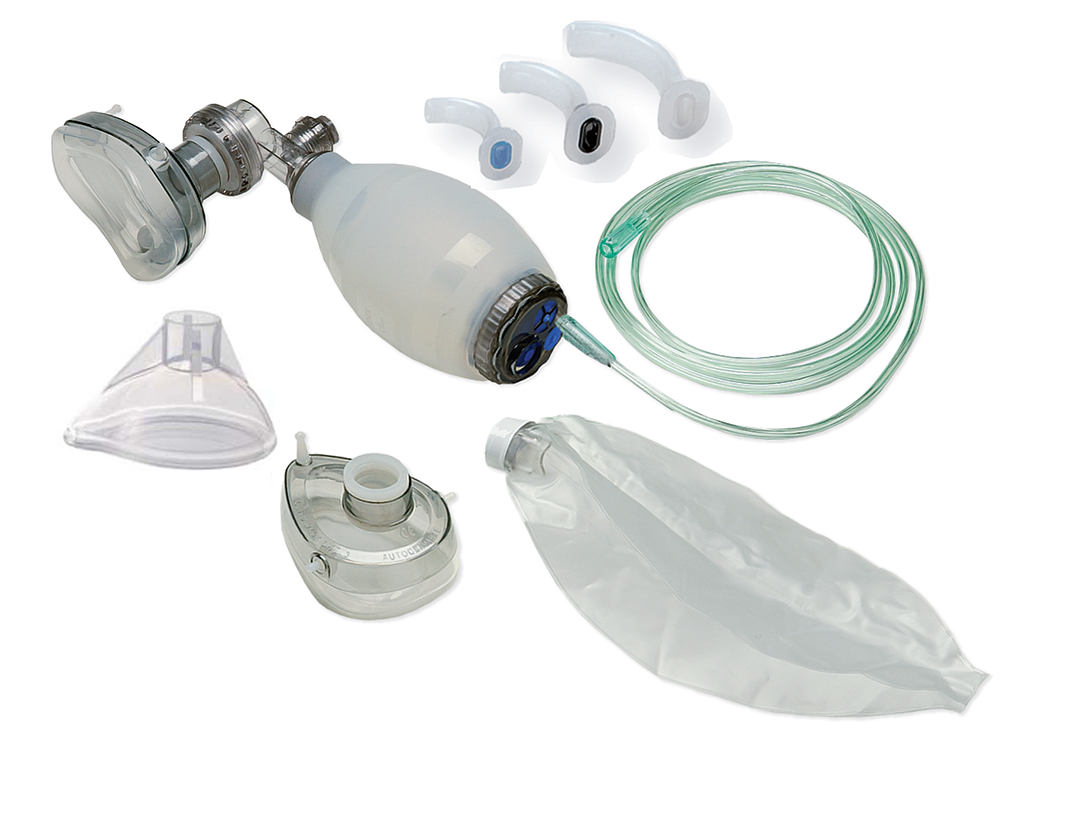
Manual operated resuscitator for chilren between 7 and 30 kg, including three different sizes face masks, an oxygen resevoir bag with required tubing to connect to oxygen supply, and three different sizes of airway guedels (Oropharyngeal airway).
Indicative Price 20.13 USD
GENERAL DESCRIPTION
Resuscitator intended for children between 7 and 30 kg.
INTENDED USE
Resuscitators are used for ventilation assistance in critical situations of respiratory distress or failure. It is considered essential equipment for health structures and emergency rescue team (to be available in emergency room, delivery room, operating theatre, intensive care unit, ambulance, etc.).
Guidance and instruction materials have been made available at no cost by the American Academy of Pediatrics via their website:. The Helping Babies Breathe guidance and training materials are freely available in many different languages.
TECHNICAL SPECIFICATIONS
Hand-operated in a horizontal position.
Portable, self-inflating, reusable.
The resuscitator is autoclavable between 121°C and 134°C.
The capacity of the self-inflating ventilation bag is 500ml.
Ventilation can be done with ambient air or with oxygen.
The resuscitator has an inlet valve with a nipple for oxygen tubing.
The resuscitator is equipped with a non-rebreather valve.
An oxygen reservoir bag should be included with a capacity between 600-2 ,600 ml.
Resuscitator can be fully disassembled, is easy to clean, disinfect, and reassemble.
The resuscitator is equipped with a pressure release (limiting) valve which safeguards against generating ventilation pressures over 45 cmH₂O, while still allowing for an airway pressure of at least 30 cmH₂O.
The pressure release valve should allow to be overridden by the user when higher pressures need to be generated in the airways.
When the pressure release valve is not overridden by the user, it should either automatically return to its default position whereby it protects against excess pressures, or be clearly visible so there is no doubt as to its closed position, even during an emergencies. To ensure users are fully aware that the limiting valve is in closed position.
All parts manufactured from high-strength, long-life materials that require no special maintenance or storage conditions.
The resuscitator, all its components and accessories are free of natural rubber latex.
The (intake, non-rebreather) valves and nipple are made from polycarbonate/polysulfone or any other material fulfilling ISO 10651-4 or equivalent.
The masks made are of silicone rubber or any material fulfilling ISO 10993-1:2018; ISO 10993-5:2009; ISO 10993-10:2013 or equivalents.
The self-inflating ventilation bag is made of transparent or translucent silicone rubber or any material fulfilling ISO 10651-4 or equivalent.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x Plastic protective pouch which can contain the full set.
1 x Masks, transparent, for infant, face shape type, size 2.
1 x Masks, transparent, for child, face shape type, size 3
1 x Masks, transparent, for, large child/teenager, face shape type, size 4.
1 x Oxygen reservoir bag with a capacity between: 600-2,600 ml.
1 x Required tubing and other accessories to connect to oxygen supply.
1 x Airway Guedel, size 0, diameter 50mm.
1 x Airway Guedel, size 1, diameter 60mm.
1 x Airway Guedel, size 2, diameter 70mm.
LIFE SPAN
The life span of the device is minimum 3 years or 20 autoclave sessions, whichever comes first.
EXPIRY DATE
This product has a minimum total shelf life of 5 years.
WARRANTY
Two years from shipping date
ENVIRONMENTAL CONDITIONS
- Operating conditions: -18°C - 45°C / 15% – 90% RH
- Storage conditions: -30°C - 50°C / 40% – 90% RH
WEIGHT AND VOLUME
Weight: 0.7 kg
Volume: 4.056 dm³
ESTIMATED DELIVERY LEAD TIME
90 days
INSTALLATION REQUIREMENTS
No installation requirements, only simple assembly which can be carried out by user.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS
S0845009, Resuscitator,hand-oper.,neonate,set
S0845152, Resuscitator,hand-oper.,adult,set
S0845006, Bulb, suction, neonatal, reusable
S0845007, Resuscitator, upright, neonate, set
COMPONENT OF A KIT
S9908402 - Resuscitation kit, basic.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements for regulatory purposes.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIa device
SAFETY & PRODUCT STANDARDS
ISO 10651-4:2009: Lung ventilators - Part 4: Lung ventilators -- Part 4: Particular requirements for operator-powered resuscitators (EN 13544-2 implied).
ISO 14971:2012 Medical devices – Application of risk management to medical devices
ISO 5356-1:2015 Anaesthetic and respiratory equipment – Conical connectors – Part 1: Cones and sockets.
ISO 15223-1 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements.
NOMENCLATURE
- GMDN code: Pulmonary resuscitator, manual, reusable (17591)
- UMDNS code: Resuscitators, Pulmonary, Manual (13367)
Related Products