S0002034
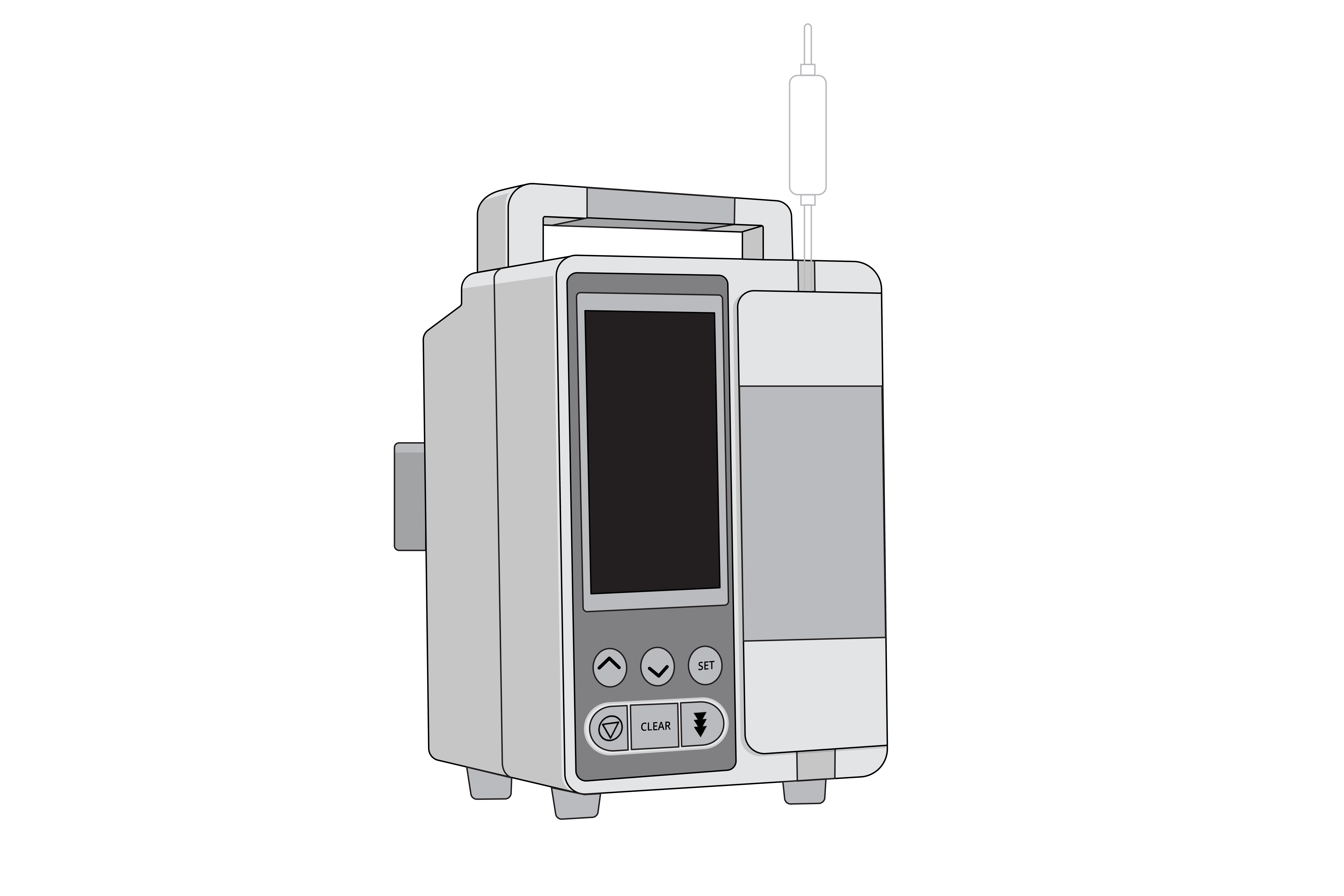
Infusion pump,with accessories
Volumetric single channel infusion pump. User programmable for infusion volume VTBI (Volume To Be Infused), time and flow rate. AC and battery powered including accessories.
Indicative Price 459.00 USD
GENERAL DESCRIPTION
Volumetric single channel infusion pump, AC and battery powered including accessories.
INTENDED USE
Infusion pumps are used to accurately deliver liquids through intravenous (IV) or epidural routes for therapeutic and/or diagnostic purposes. They are used in hospitals, in alternative care settings (e.g., homes, long-term care facilities, physicians' offices, outpatient infusion centres), and, occasionally, in emergency medical service vehicles.
In general, infusion pumps are used when the solution to be administered must be delivered with greater accuracy than can be provided through a manually adjusted gravity administration set.
TECHNICAL SPECIFICATIONS
Automatic calculation of third parameter when user enters other two parameters (volume, time, and flow rate).
Minimum guaranteed flow rate range of 1-1500 mL/hr in either 0.1 or 1 mL/hr increments.
Keep Vein Open (KVO) rate of 1-5 mL/hr.
The accuracy of the flow rate should be 5% or better.
Robust design allow use in demanding environments, resistant against hospital-grade cleaning solutions, fluid proof.
Capable of being mounted on mobile pole/(roll) stand, bed rail and wall-mounted rail.
Open system, compatible with wide range brands of giving sets. Unit is to be calibrated to the characteristics of a specific brand infusion set through DIP switches prior to using the unit.
Designed for frequent and easy dismount and disinfection with hospital-grade products
Built in battery depending on the model either a lithium ion or a lithium metal hydride.
Battery life lasts at least 4 hours at 25mL/hr flow rate.
Automatic switch from mains to battery during power failure.
Auto-off when not in use.
Power requirements: 240 Volts – 50 Hz (110 Volts – 60 Hz available on request, indicate when ordering).
DISPLAY FEATURES.
An integrated display indicating following parameters/information:
- Alarms.
- Pumping status.
- Volume infused.
- Volume limit/Volume To Be Infused (VTBI).
ALARMS AND SAFETY FEATURES
Alarms are audible and visual.
Ability to silence audio alarms for maximum of 2 minutes.
The following alarms are included:
- Air-in-line alarm.
- Down-stream occlusion alarm.
- Open door alarm.
- Infusion complete notification.
- Low/depleted battery alarm.
- Incorrectly loaded set alarm.
- The unit should be protected against uncontrolled gravity flow, a so-called free-flow protection.
- The unit is equipped with a "control lock-out" feature, preventing tampering by patients or visitors with the controls of the unit. (depending on the supplied model).
SUPPLIED WITH
Instructions for assembly, use and maintenance in English.
1 x Plastic protective dustcover.
1 x Start-up set of 30 giving sets.
1 x Spare battery pack.
1 x Mounting bracket required for fixation to mobile pole/(roll) stand, bed rail and wall-mounted rail.
1 x pole/stand, option to roll.
1 x Set of spare fuses, if required.
ESTIMATED LIFE SPAN
5 years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 10°C - 30°C / 30% - 75% RH.
- Storage conditions: -20°C - 55°C / 20% - 90% RH.
- Atmospheric pressure: 700 ~ 1060 hPa.
- Ingress protection rating: IPX3.
WEIGHT AND VOLUME
Weight: 12.896 kg.
Volume: 53.33 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS
S0002017, Syringe pump,with accessories
S0531991, Infusion giving set,sterile,s.u.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-1-8 Medical electrical equipment - Part 1-8: General requirements for basic safety and essential performance - Collateral Standard: General requirements, tests, and guidance for alarm systems in medical electrical equipment and medical electrical systems
- IEC 60601-2-24 Medical Electrical Equipment – Part 2-24: Particular requirements for the safety of infusion pumps and controllers.
NOMENCLATURE
- GMDN code: General-purpose infusion pump, line-powered (13215), Infusion Pumps, Multitherapy, Large Volume (28057).
- UMDNS code: Infusion Pumps, Multitherapy, Large Volume, Single-Channel (27889).
accessories
Related Products