S0002059
Defibrillator,basic,w/access
External defibrillator for adult and paediatric patients, with ECG monitor and printer, synchronised and bi-phasic, portable, AC andbattery powered, with accessories
Indicative Price 4,357.80 USD
GENERAL DESCRIPTION
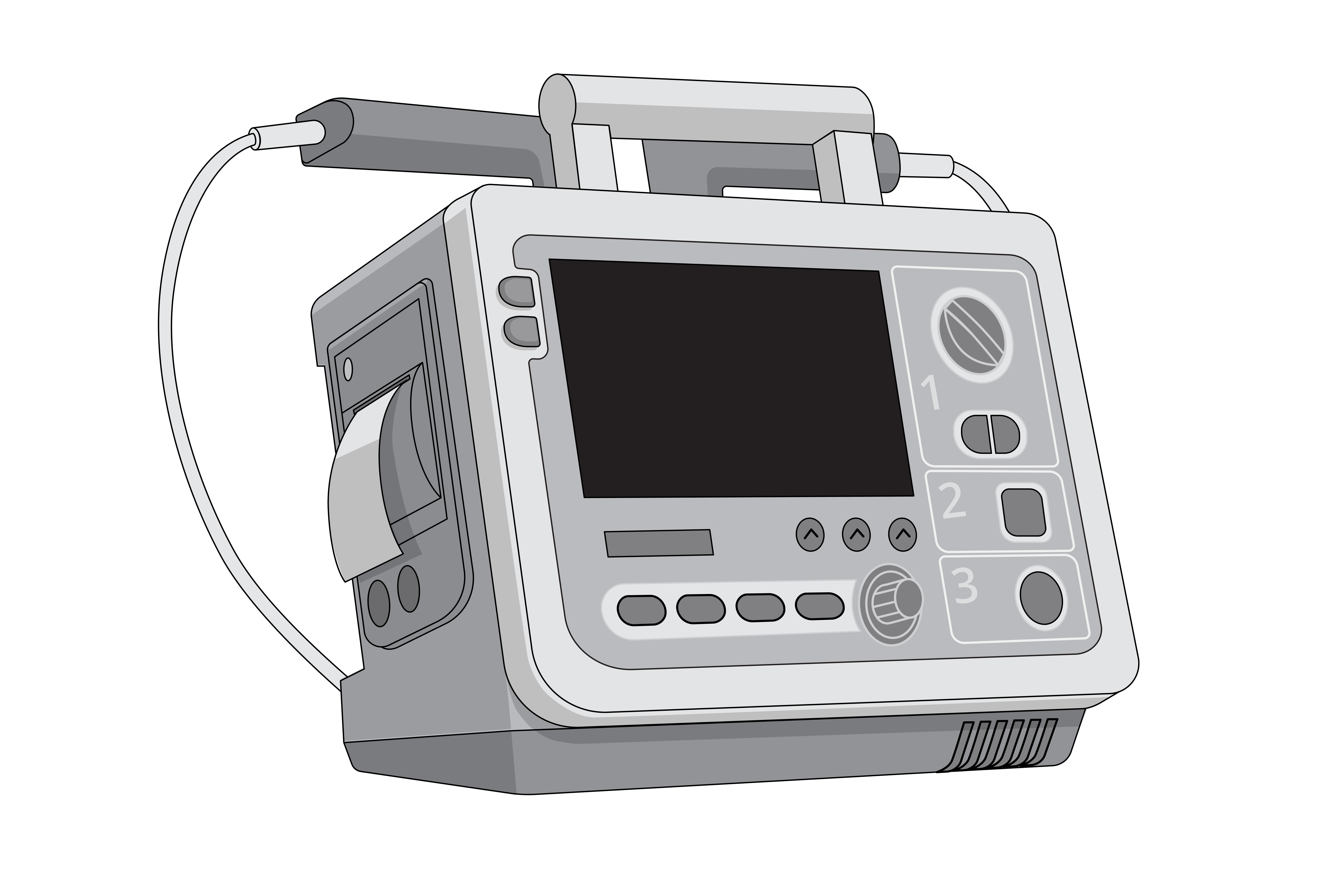
External defibrillator with ECG monitor for adult and paediatric patients.
INTENDED USE
Defibrillators deliver a high-voltage electrical impulse to the heart in order to restore normal rhythm and contractile function in patients who are experiencing ventricular fibrillation (VF), ventricular tachycardia (VT), or another shockable rhythm. An ECG monitor included with the unit is used to verify the presence of a shockable rhythm and the effectiveness of treatment.
TECHNICAL SPECIFICATIONS
Suitable for adult and paediatric patients.
Supplied with hand-held paddles suitable for adults and paediatric patients.
DEFIBRILLATOR FEATURES
Biphasic energy waveform.
Manual defibrillation ("defib") and synchronized cardioversion ("sync") modes.
Adjustable energy output range of 2 - 50 J for paediatric patients.
Adjustable energy output range for adult patents 50 - either 200 or 360 J for adult patients.
Minimum of 19 steps between minimum and maximum energy output.
Paddle controls for charging and discharging.
Automatic impedance compensation.
Integrated backlit, flat panel display, minimum diagonal measurement of 12.7 cm (5 inches).
Maximum time from charge to shock ready < 8 s (through mains power and battery power).
Maximum time from power on to shock ready < 15 s (through mains power and battery power).
Internal safety discharge feature (if shock is not delivered).
ECG MONITOR
Three (3) lead ECG configuration (I, II and III) and cable.
ECG waveform display.
Waveform display sweep speed of 25 mm/sec.
Sensitivity settings (ECG gain) must include at least: 5, 10, 20 and 40mm/mV.
Adjustable ECG print modes and settings (real-time, manual).
Lead fault indicator.
PRINTER, STORAGE AND DATA
Built-in 3-channel thermal printer.
Prints ECG waveform, events, settings, and alarms.
Paper speed of 10, 25 and 50 mm/sec.
Internal memory storage for ECG, events (16 hours ECG monitoring) and parameters.
DISPLAY
The display shows following parameters:
- Defibrillation mode.
- Energy level.
- Ready/charging state.
- ECG waveform.
- Heart rate.
- Date and time.
- Settings.
- Mains and battery status.
- System messages.
ALARMS AND SAFETY
Below listed alarms are audible as well as visual:
- Paddle and ECG electrode connection.
- Mains and battery status.
- System errors.
The unit include automatic self-testing of paddles, ECG electrodes, the discharge function, battery and mains power, printer, and paper status, and for other device errors.
BATTERY AND POWER
Removable rechargeable battery.
Integrated battery charger and transformer.
Automatic switch to battery in case of power failure and battery charge on connection to mains power.
Operating time on battery: > 130 discharges at 200 J or > 2 hours continuous ECG monitoring.
Maximum battery recharge time less than 4 hours.
Battery type Nickel-Metal Hydride or Lithium Ion (depending on the model supplied).
Power requirements: 100 - 240 Volts - 50/60 Hz.
EXTERIOR DESIGN:
Integrated carry handle.
Maximum weight including paddles and battery < 6.1 kg.
Designed for frequent and easy dismount and disinfection with hospital-grade products.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English.
1 x pair of adult paddles.
1 x pair of paediatric paddles/adapters.
1 x ECG patient cable.
3 x pack of 100 single-use ECG electrodes.
3 x 100 ml bottles of conductive gel.
1 x spare rechargeable battery.
5 x rolls/packs of thermal paper.
ESTIMATED LIFE SPAN
10 years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 0°C - 45°C / 10% – 95% RH.
- Storage conditions: -20°C - 60°C / 10% – 95% RH.
- Altitude: 0 to 3,000m.
- Ingress protection rating: IP33.
WEIGHT AND VOLUME
Weight: 20.00 kg.
Volume: 101.00 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS
S0002062, ECG recorder,portable,w/access.
ALTERNATIVE PRODUCTS
S0002058 Defibrillator,AED,w/access.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class III device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-2-4:2010+AMD1:2018 CSV Consolidated version Medical electrical equipment - Part 2-4: Particular requirements for the basic safety and essential performance of cardiac defibrillators.
NOMENCLATURE
- GMDN code: Manual external defibrillator (37806).
- UMDNS code: Defibrillators, External, Manual (11134).
Related Products