S0002064
Incubator,automatic,basic,w/access
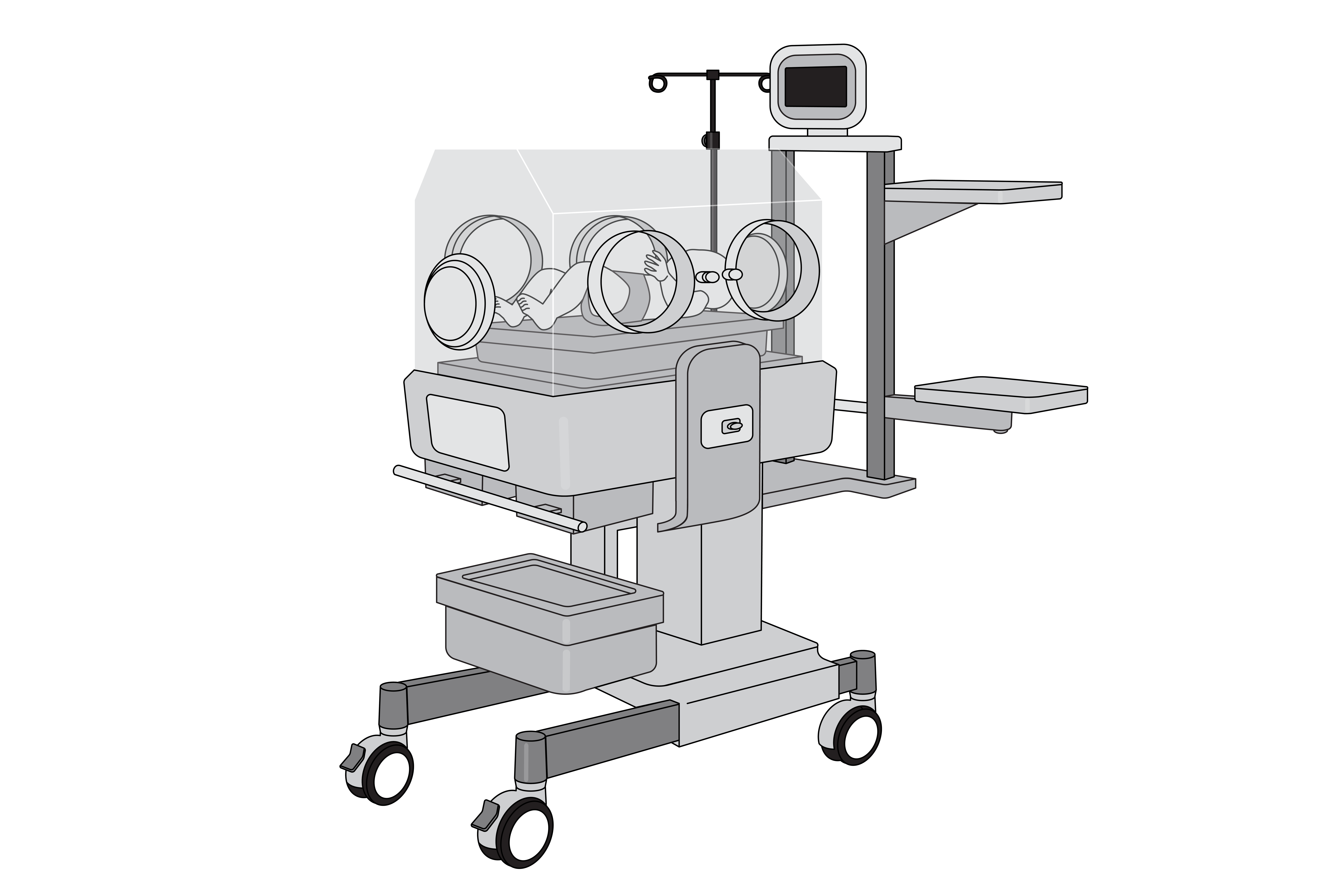
Neonatal incubator, double-wall, electronic, automatic, mobile, with accessories
Indicative Price 6,388.27 USD
GENERAL DESCRIPTION
Neonatal incubator, double-wall canopy, mobile.
INTENDED USE
An infant incubator provides a closed, controlled environment that warms an infant by circulating heated air over the skin. The heat is then absorbed into the body by tissue conduction and blood convection. Ideally, both the skin and core temperatures should be maintained with only minor variations. Incubators feature better temperature control than radiant warmers (overhead heating units), which can overheat or underheat the infant. However, an infant in an incubator is less accessible to the caregiver.
TECHNICAL SPECIFICATIONS
The unit is equipped with an automatic/servo-controlled air and skin temperature control.
Microprocessor-based system for temperature and humidity regulation.
Air and skin temperature control modes use thermistor-based automatic servocontrol.
Air-mode set temperature range is 23-37°C in 0.1°C increments.
Automatic self-test on power up.
Performs internal component diagnostics at regular intervals during operation.
Includes a visual heater power indicator.
Internal backup thermistor.
Includes built-in water reservoir for additional air humidity.
Maximum internal sound level during normal operations is ≤ 60 dB; during audible alarms ≤ 65 dB.
Power requirements either 110 Volt / 60 Hz or 220 Volts – 50 Hz to be specified at the time of ordering.
CONSTRUCTION AND DESIGN
Has a double-walled clear canopy.
Latched door on front panel, at a minimum 30 cm high x 75 cm wide.
Minimum of four latched hand access ports and six tube access ports.
Fixed mattress tray that tilts between 0° and at a minimum up to ± 12°
Mattress with removable washable and fluid-resistant cover, minimum dimensions 65 cm long x 37 cm wide.
Display shows operational status, with set and measured values and alarms.
Includes at least one equipment shelf, IV pole and side rails for mounting accessories and equipment.
Base includes integrated cabinet with at least two drawers.
Side handles to facilitate positioning.
Four (4) anti-static swivel castors that are at least 10 cm in diameter.
A minimum of two (2) the castors are equipped with brakes.
SERVO AIR MODE SET TEMPERATURE:
Within the range of 25 - 37°C, never lower than 20°C, adjustable in 0.1°C increments, and with an accuracy equal to or better than +/-0.8°C.
Should include an override up to 39°C.
SERVO SKIN MODE SET TEMPERATURE RANGE:
Temperature alarms can be set for a minimum range of 34 - 37°C, but never lower than 32°C, adjustable in 0.1 °C increments, with an override up to 38°C and an accuracy equal to or better than +/-0.5°C.
ALARM FUNCTIONALITIES
Alarms are both audible, as well visual.
Alarms are available for:
- High/low skin temperature.
- High/low air temperature.
- Air fan failure.
- Temperatures exceeding 39°C in any mode.
- Temperature probe failures and/or disconnects.
- Power failure alarms.
- Alarm if a circuit fault has been detected.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x Plastic protective dustcover.
3 x Set of spare bacterial air filters.
1 x Set of spare fuses.
OPTIONAL ACCESSORIES AVAILABLE
At extra costs additional optional features can be requested:
- Air/oxygen blender.
- Integrated weighing scale.
- LCD touch screen.
- Electric height adjustment with foot pedals.
ESTIMATED LIFE SPAN
Between 8 – 10 years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 10°C - 40°C / 30% - 90% RH.
- Storage conditions: -10°C - 70°C / < 90% RH.
- Atmospheric pressure: 700 ~ 1060 hPa.
WEIGHT AND VOLUME (packaged)
Weight: 150 kg.
Volume: 2,000.00 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS:
S0002031, Monitor,patient,portable,w/access.
S0002033, Pulse oximeter,portable,w/access.
S0002034, Infusion pump,with accessories.
S0002017, Syringe pump,with accessories.
S0002032, Photo therapy unit,w/access.
S0002018, Phototherapy irradiance meter.
ALTERNATIVE PRODUCTS
S0002035, Table,resusc,newborn,w/access.
S0002060, Warmer system,newborn, basic, set.
S0002065, Warmer system,newborn,radiant,w/access.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-2-19:2009+AMD1:2016 Part 2-19: Particular requirements for the basic safety and essential performance of infant incubators.
NOMENCLATURE
- GMDN code: Conventional infant incubator (36025)
Related Products