S0004222
Warmer,newborn,portable,conductive
Neonatal warming system, portable with phase change phase material, for conductive heating in settings with intermittent availability of electricity.
Indicative Price 638.00 USD
GENERAL DESCRIPTION
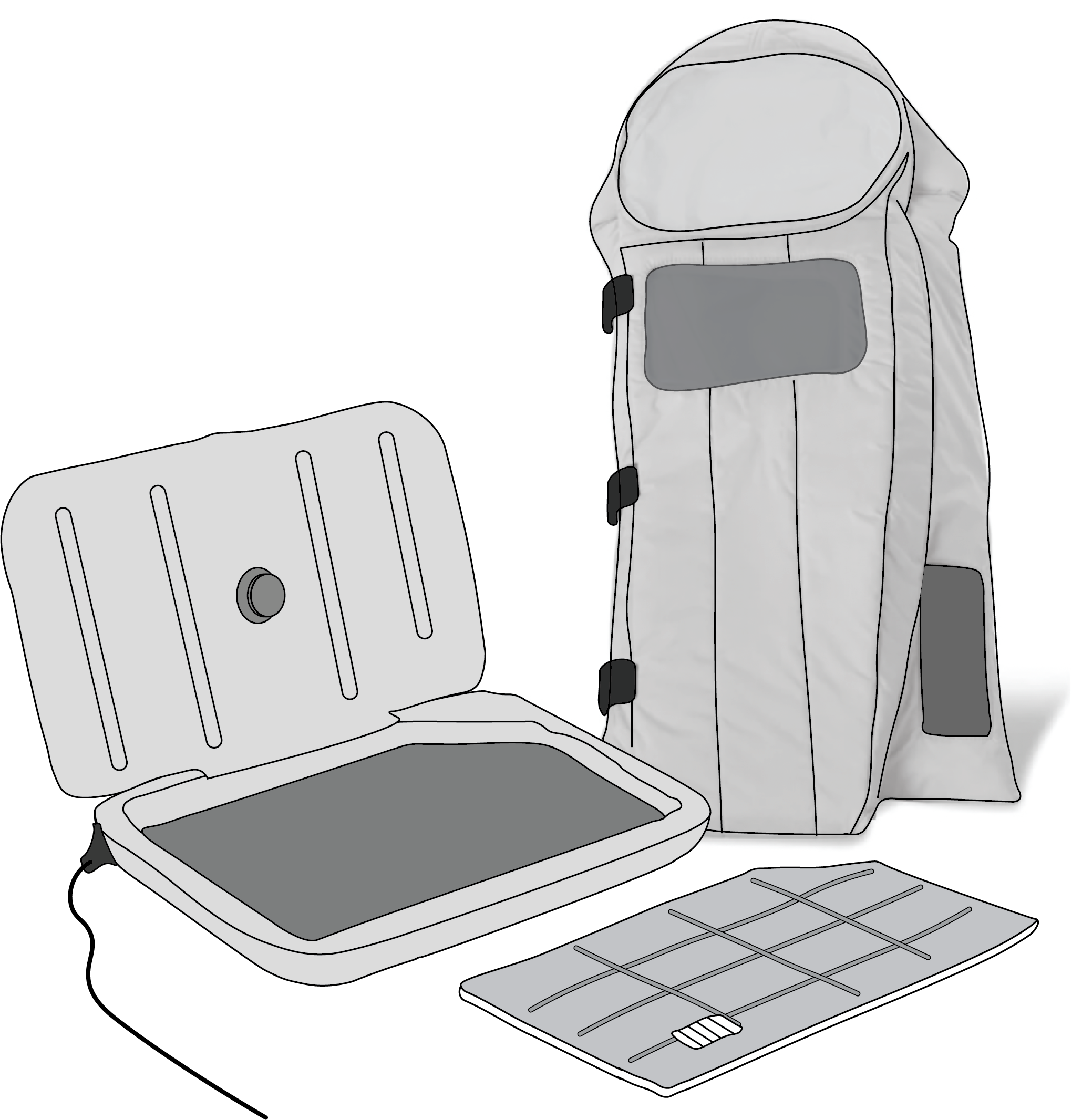
A portable infant warmer using a phase change material (PCM) based warming system, chemically engineered to maintain a constant temperature for several hours once heated, including a phase change material insert, external warming oven and warming nest wrap.
INTENDED USE
Low body temperature, or hypothermia, is a significant contributor to neonatal mortality, with a body temperature below 36°C at birth identified as an independent risk factor for death among pre-term infants. A portable warmer uses a PCM insert, and a warming nest wrap to raise or maintain the body temperature of newborn and premature babies through conductive heating in settings with intermittent availability of electricity, as in patient transport during referral between health facility levels or within a health facility. Consistent monitoring and care should be provided as a patient in a portable warmer is less accessible to the caregiver.
TARGET POPULATION
Newborn and premature babies and infants during patient transport.
TECHNICAL SPECIFICATIONS
A portable infant warmer using a phase change material (PCM) based warming system, chemically engineered to maintain a constant temperature for several hours once heated, including a phase change material insert, external warming oven and warming nest wrap.
Intended to provide warmth to newborns weighing between 1.5 to 2.5 kilograms.
For use by clinically trained caregivers as an infant warmer in patient transport during referral between health facility levels or within a health facility.
Heat time approximately 40 minutes under 25°C ambient temperature.
Portable assembled product with PCM insert in warming nest wrap weighing under 1.7 kg, ensuring easy transport and care for the newborn patient.
CONSTRUCTION AND DESIGN
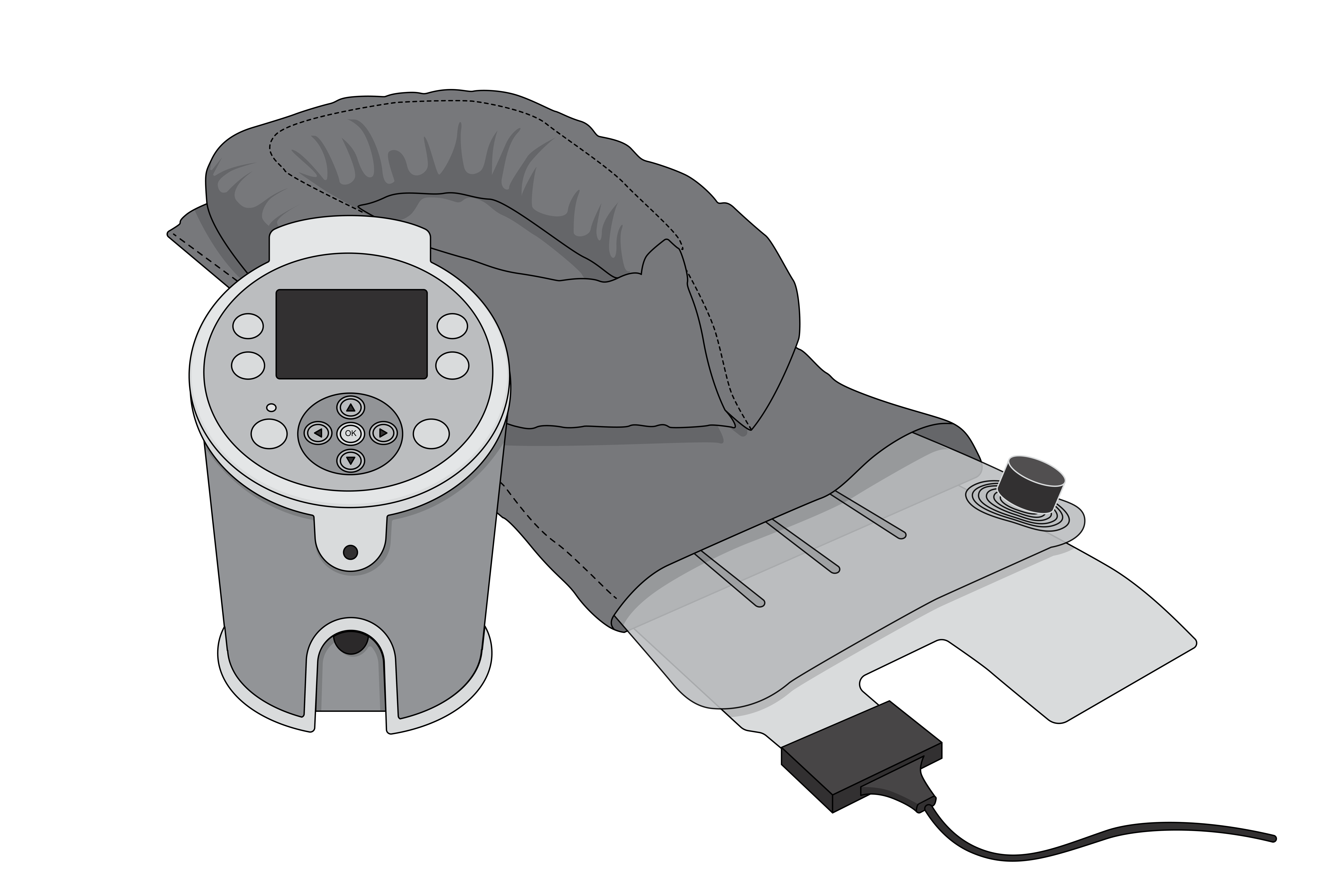
Phase Change Material (PCM) Insert
Heat resistant polyurethane PCM insert heated in an external warming oven; melts and stores latent heat in a physical process involving no chemical change.
Water layer to eliminate hot spots and provide uniform heat to patient location.
Non-toxic PCM with a phase transition temperature near 37°C.
PCM insert once heated maintains a 34.5 to 38°C environment for 4 hours at the patient location under 25°C ambient temperature.
Visible non-electric liquid crystal temperature indicator that indicates usable temperature range in three states (too hot / ok / too cold) with a moving line (PCM temperature indicator).
Warming Nest Wrap
Reusable warming nest wrap with compartment for PCM insert that is separate from, but in thermal contact with the newborn patient.
Insulated hood and wrap to control head and body heat loss.
Patient compartment made of fluid-proof, bacteria-resistant, skin-safe thermoplastic polyurethane fabric with a minimal seam design.
Accessible design with flaps for patient placement and adjustable straps to secure the wrap for patients of different sizes.
Clear observation window to facilitate monitoring of breathing, skin colour and general movement.
Compatible with oxygen and IV lines.
Clear observation window to monitor the PCM temperature indicator.
External Warming Oven
Simple operation, AC powered oven with alternative power compatibility (e.g., generator, solar power).
Automatic self-diagnostic on start-up.
Control mechanism to ensure the PCM insert is heated to operating temperature (between 37°C and 40°C).
Automatic recommencement of heating in case of loss of power supply or outage.
Heating element to withstand up to 1.5 kV breakdown voltage.
Grounded aluminium warming plate.
ERROR INDICATORS
External warming oven functionality alarms are both audible and visual.
Visual PCM temperature indicator for PCM insert that indicates usable temperature range in three states (TOO HOT / OK / TOO COLD) with a moving line.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x plastic protective dustcover.
1 x warming nest wrap.
1 x phase change material insert.
1 x external warming oven.
1 x set of spare fuses, if applicable.
SHELF LIFE
Phase change material insert: 1 year.
ESTIMATED LIFE SPAN
External warming oven: 8 years.
Warming nest wrap: 8 years.
Phase change material insert: 1 year.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
Operating conditions: 10°C to 34°C / ≤ 75% RH.
Storage conditions: -20°C to 60°C / ≤ 90% RH.
Atmospheric pressure: 50 kPa to 106 kPa.
Ingress protection rating: IP20.
WEIGHT AND VOLUME (packaged)
Weight: 5 kg
Volume: 36.90 dm3
UTILITY REQUIREMENTS
Intermittent supply of electricity (110-230V, 50/60 Hz, 130W/550W) as needed to warm the phase change material insert.
ESTIMATED DELIVERY LEAD TIME
120 Days
INSTALLATION REQUIREMENTS
Assembly and commissioning this product by a qualified technician is recommended.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals and standard protocols for infection prevention and control.
RELATED PRODUCTS:
S0002035, Table,resusc,newborn,w/access.
S0002060, Warmer system,newborn, basic, set.
S0002065, Warmer system,newborn,radiant,w/access.
COMPONENT OF A KIT
No part of a kit.
LABELLING
PRIMARY PACKING
In accordance with EU MDR 2017/745 Annex I, Chapter III, point 23.2. Follow below hyperlink for details on this regulation.
https://eur -lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32017R0745 &qid=1622012817907&from=EN#d1e32-94-1
SECONDARY PACKING
- Labelling on the packaging unit: Labelling to be the same as primary packaging.
- Extra information required: Number of units per box.
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
Manufacturer is certified for ISO 9001 Quality management systems – Requirements.
REGULATION, CONFORMITY REQUIREMENTS & CLASSIFICATION
Classified under EU MDR 2017/745 as Class I device.
SAFETY & PRODUCT STANDARDS
IEC 60601-1: Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
IEC 60601-1-2: Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
IEC 60601-1-8: Medical electrical equipment — Part 1-8: General requirements for basic safety and essential performance — Collateral standard: General requirements, tests and guidance for alarm systems in medical electrical equipment and medical electrical systems.
IEC 62304: Medical device software — Software life cycle processes.
IEC 10993-1: Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process.
IEC 62366-1: Medical devices — Part 1: Application of usability engineering to medical devices.
NOMENCLATURE
GMDN code: Infant warmer (17433).
UMDNS code: Warmers, Radiant, Infant, Transport (13251).
Related Products