S0686000
Stethoscope,binaural,complete
Stethoscope, binaural, with two diaphragms for dual-use (adult and paediatric auscultation) including spare diaphragms for adults and children.
Indicative Price 5.40 USD
GENERAL DESCRIPTION
Stethoscope, binaural, including accessories.
INTENDED USE
A mechanical listening device designed for listening to sounds from the heart, lungs, and/or gastrointestinal tract from adult and paediatric patients. It comprises a membrane at the listening head connected by a dual tube to the headgear with ear olives that are placed into the users’ ears.
TECHNICAL SPECIFICATIONS
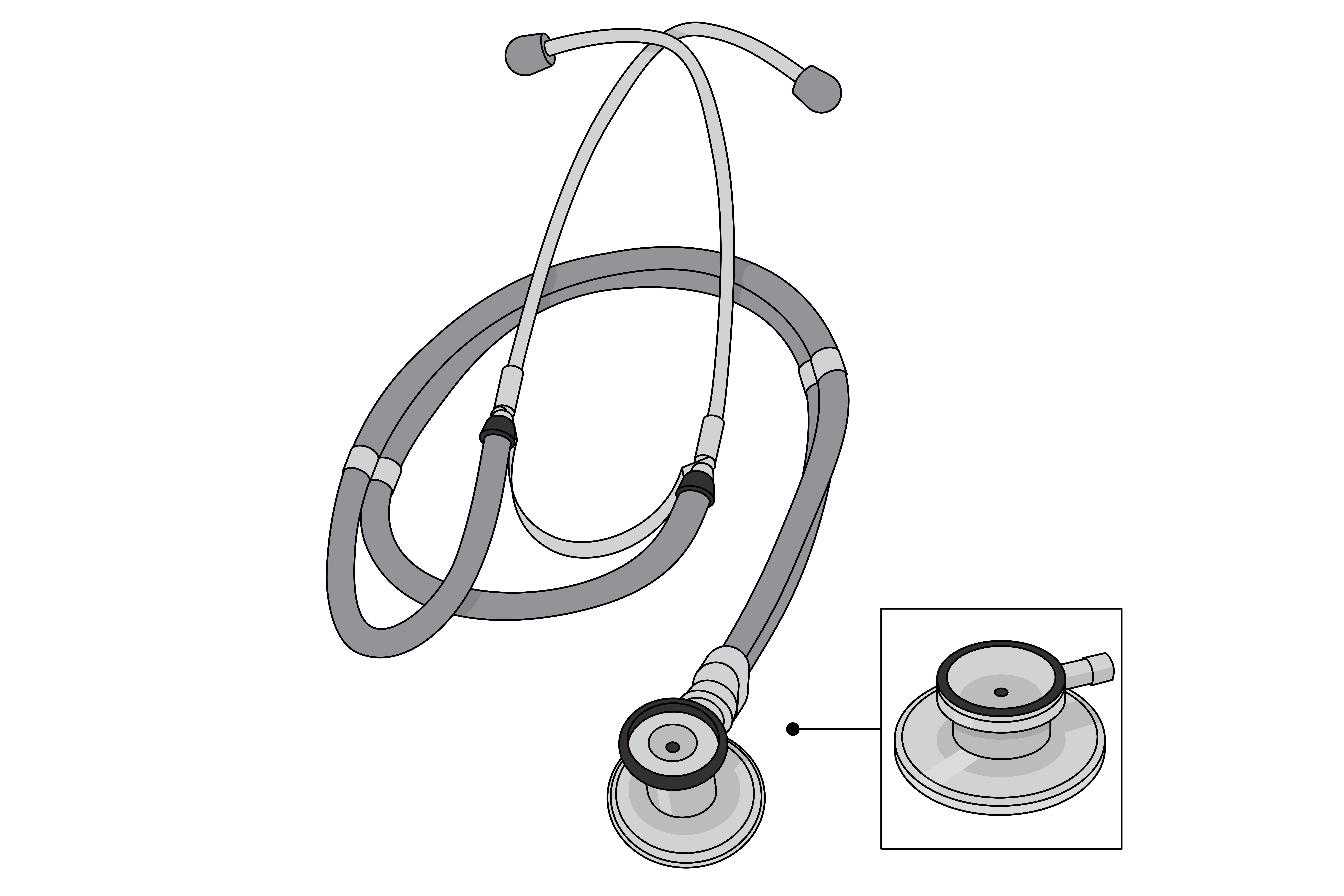
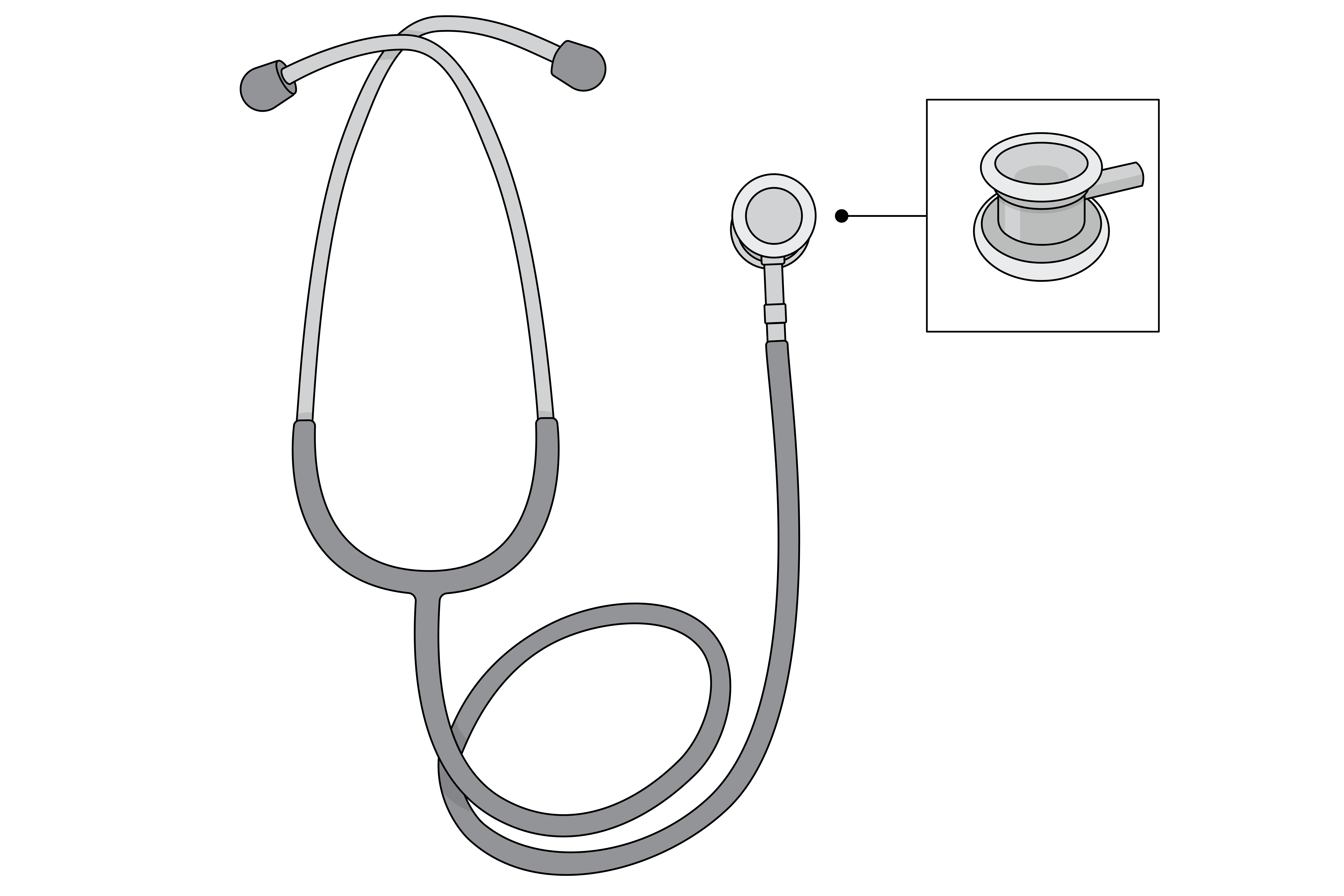
Comprises a chest piece connected by a double tube to the headgear with earpieces that are placed into the users’ ears.
Double cup, with two diaphragms for dual-use (adult and paediatric auscultation) chest piece in zinc alloy.
Adult diaphragm Ø: 45,5mm; paediatric diaphragm Ø: 31.5mm.
Tube made of PVC and is crack resistant.
Tube impervious to outside noises, guaranteeing full transmission of sound, good auditive quality.
Tube diameter: outer diameter 10mm, inner diameter 4.8mm. Tube length 560mm.
Sensitivity from 3.2dB to 26dB in a range from 50 to 1000Hz for cardiology.
Sensitivity 8.1dB in a range from 600 Hz to 1,500Hz for pneumology.
Arms: brass-steel with a flexible spring.
Removable plastic earpieces.
Latex-free.
Designed for frequent and easy disassembly and disinfection with hospital-grade products.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x spare adult diaphragm.
1 x spare paediatric diaphragm.
1 x set spare earpieces.
ESTIMATED LIFE SPAN
5 years.
WARRANTY
Two years.
ENVIRONMENTAL CONDITIONS
Storage conditions: 0 - 60°C / 90% RH.
Operating conditions: 10 - 50°C / 90% RH.
WEIGHT AND VOLUME (Packaged)
Weight: 400 gr.
Volume: 1.78 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
There are no installation or commissioning requirements.
TRAINING REQUIREMENTS
Where staff has experience with these types of devices, no user training will be required.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements.
RELATED PRODUCTS
S0686500, Stethoscope,foetal,Pinard
S0845184, Stethoscope,neonatal,binaural,complete
COMPONENT OF A KIT
S9901029 - IEHK2017,kit,suppl.2-equipment
S9902221 - Midwifery kit – equipment, 2
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDR 2017/745 as Class I device.
SAFETY AND PRODUCT STANDARDS
- EN ISO 15223-1 (EN 980) Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements.
NOMENCLATURE
GMDN Code: 13755
Related Products