S0686500
Stethoscope,foetal,Pinard
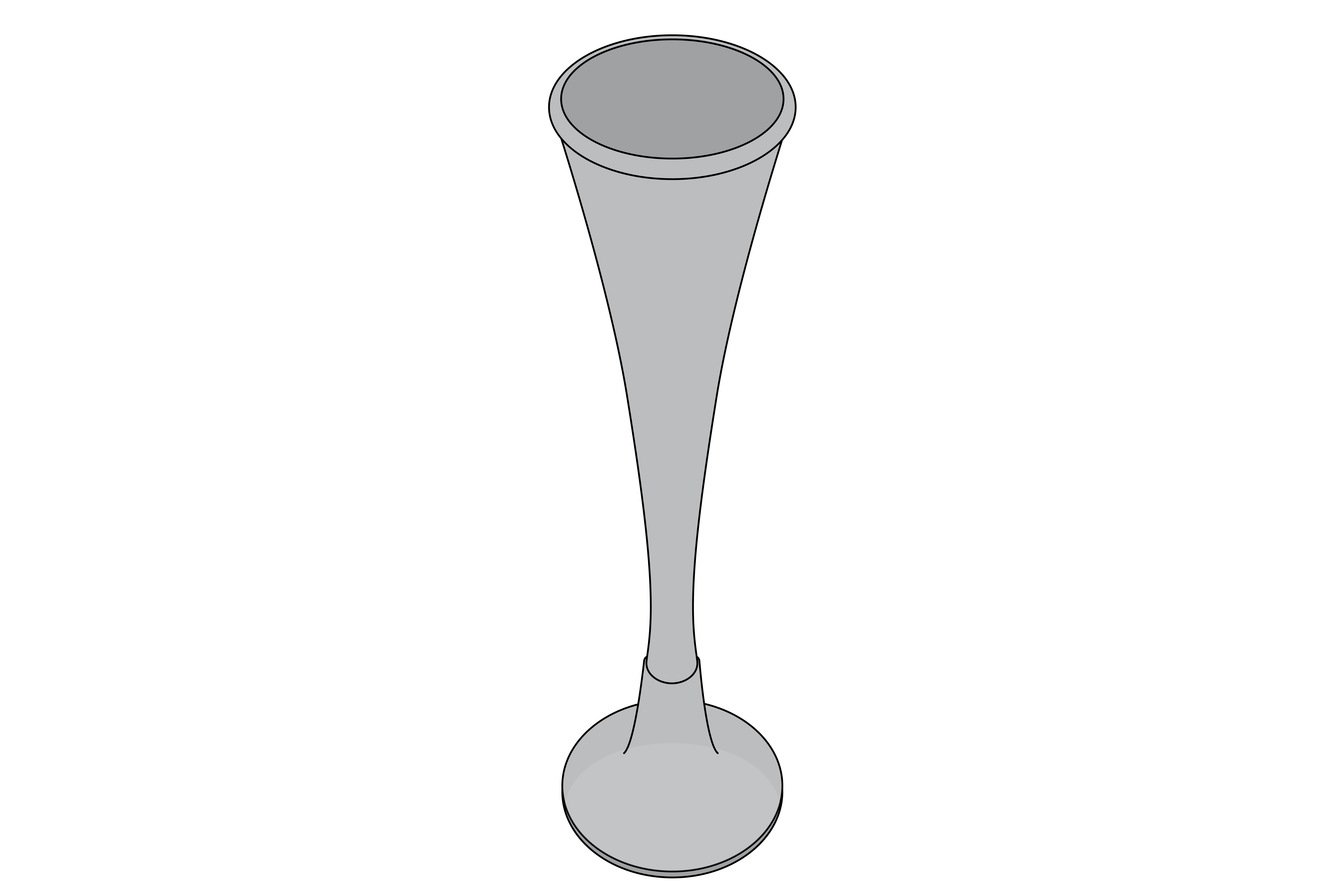
Foetal (Pinard) stethoscope used to auscultate the heart sounds of the embryo in the womb.
Indicative Price 2.35 USD
GENERAL DESCRIPTION
Stethoscope, foetal, Pinard
INTENDED USE
A mechanical listening device that is used for listening to foetal heart sounds. It is designed as a hollow tube (trumpet-shaped) to transmit the foetal heart sound through the inner channel by air conduction and is pressed against the outside of the mother's abdomen and the user will place their head and ear to the listening end of the device.
TECHNICAL SPECIFICATIONS
Small conical instrument, of which the top allows to the practitioner to place the ear, while the flat and circular basis is placed on the abdomen of the pregnant woman.
Monoaural.
Made from unbreakable plastic or aluminium.
Length between: 140 - 180 mm.
Designed for frequent and disinfection with hospital-grade products.
ESTIMATED LIFE SPAN
Five years
WARRANTY
Two years.
ENVIRONMENTAL CONDITIONS
Storage conditions: 0 - 50°C / 85% RH.
Operating conditions: 10 - 40°C / 85% RH.
WEIGHT AND VOLUME (Packaged)
Weight: 50 gr.
Volume: 0.6 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
There are no installation or commissioning requirements.
TRAINING REQUIREMENTS
Where staff has experience with these types of devices, no user training will be required.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements.
RELATED PRODUCTS
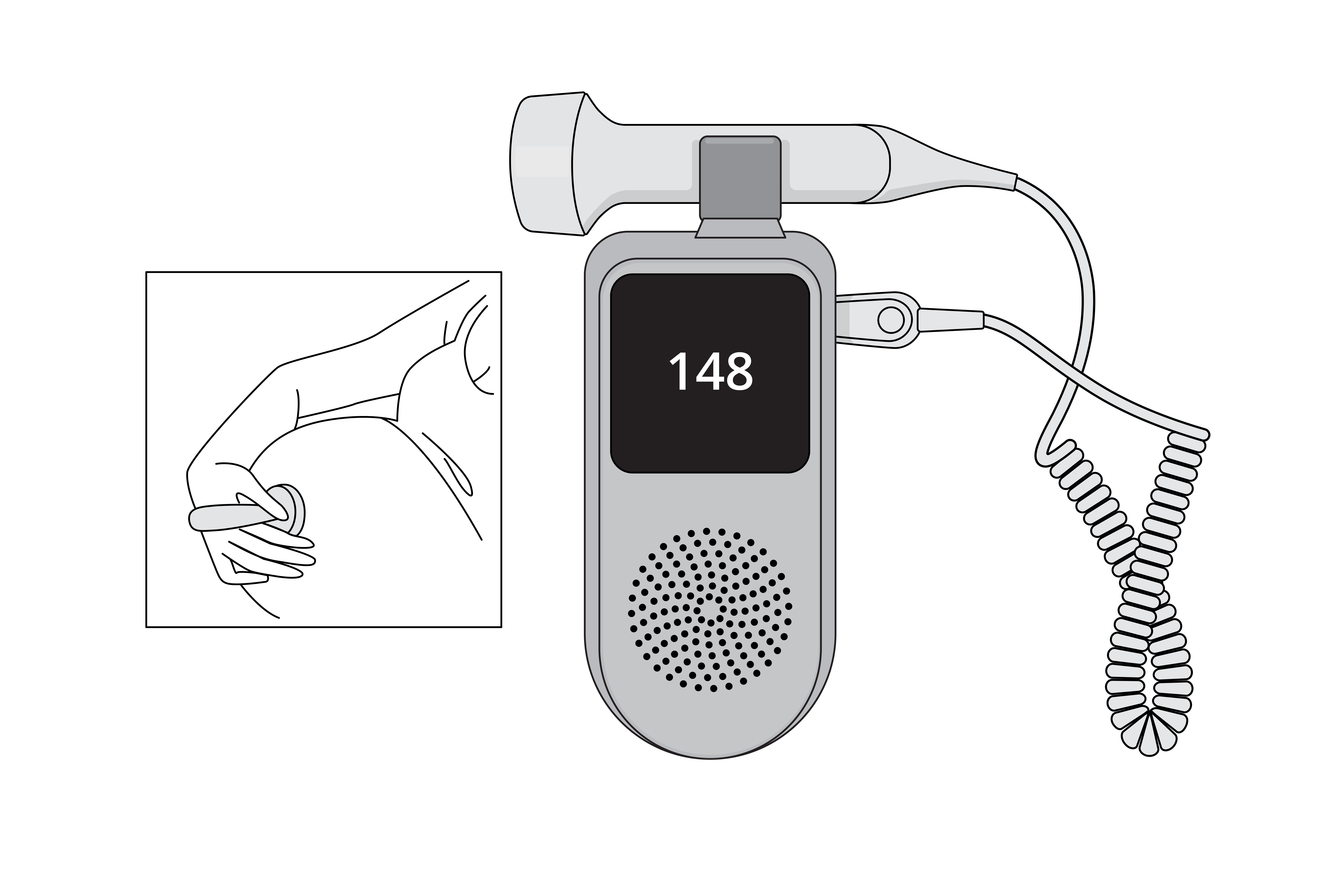
S0002061, Doppler,FHR detector,w/access
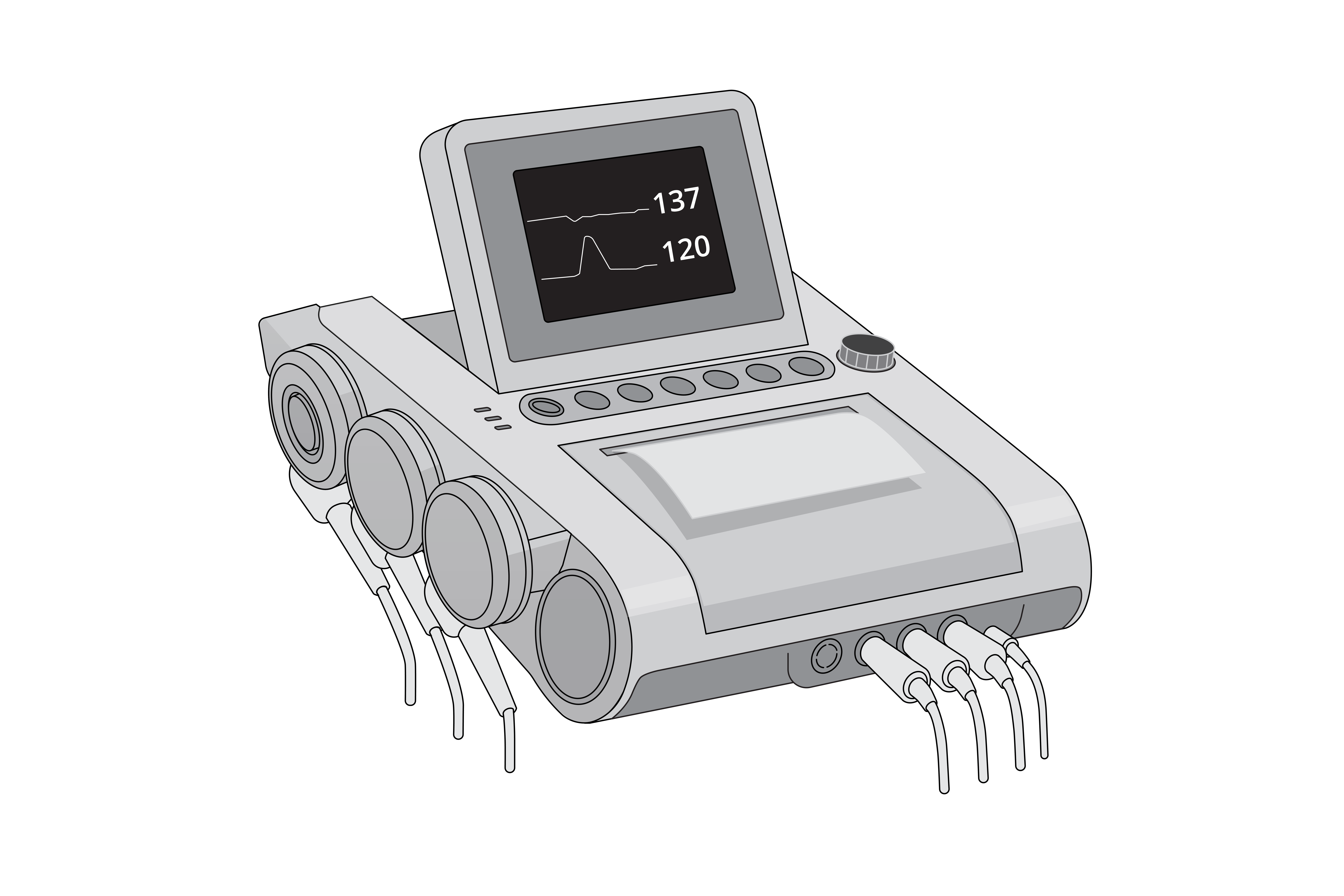
S0002069, CTG monitor,with accessories
COMPONENT OF A KIT
No
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDR 2017/745 as Class I device.
NOMENCLATURE
GMDN Code: 32659
Related Products