S0845184
Stethoscope, neonatal, binaural,complete
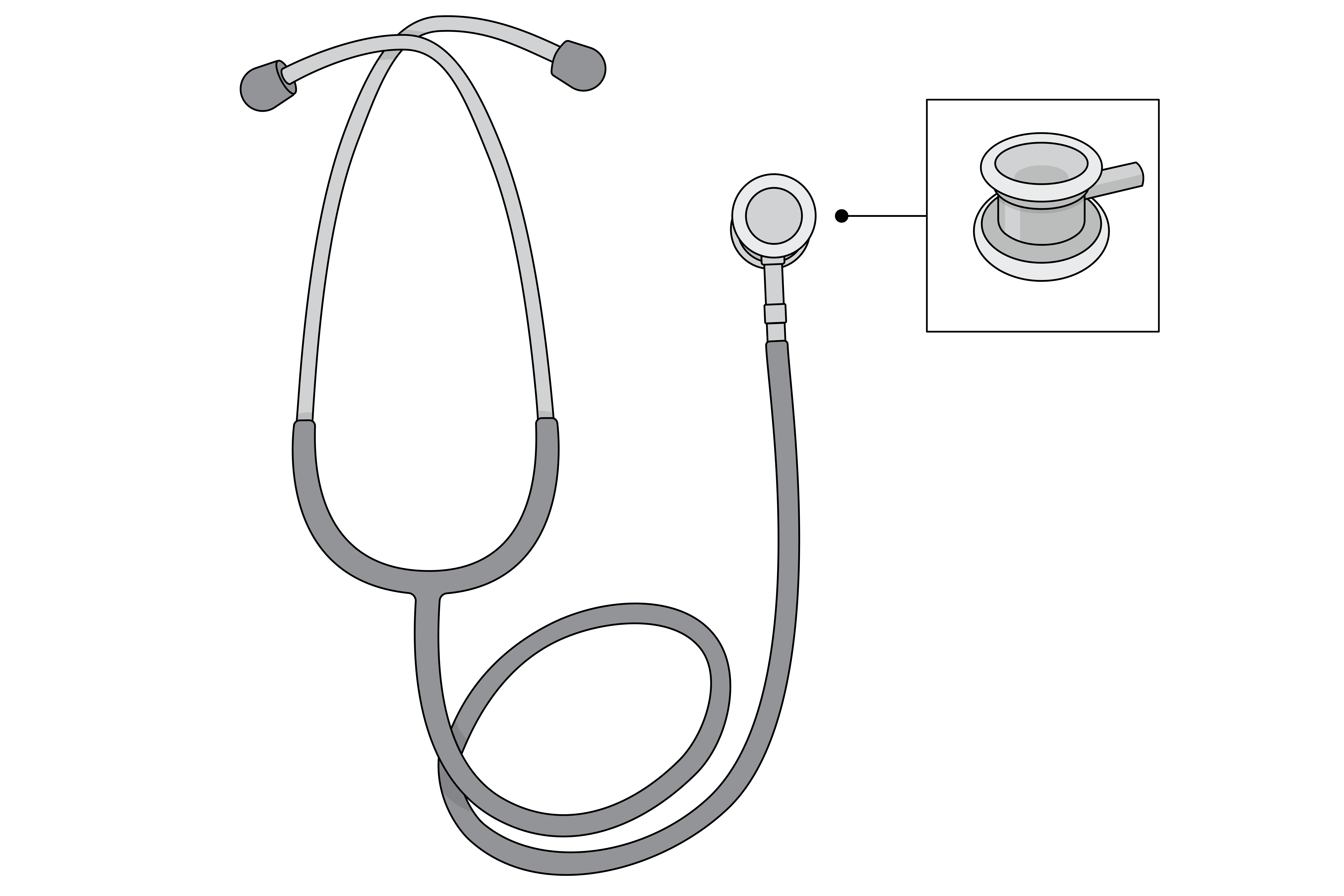
Stethoscope, binaural, with a dual head chest piece containing a diaphragm and a bell cup suitable for use with newborns and infants, comes with spare diaphragm and ear pieces.
Indicative Price 2.57 USD
GENERAL DESCRIPTION
Stethoscope for newborns and infants including accessories.
INTENDED USE
A mechanical listening device designed for listening to sounds from the heart, lungs, and/or gastrointestinal tract. It typically comprises amembrane at the listening head connected by a split "Y" tube to theheadgear with ear olives that are placed into the users’ ears. Designed specifically to detect higher frequencies which are produced by newborns and infants.
TECHNICAL SPECIFICATIONS
Double cup chest piece with one diaphragm and one bell cup in zinc alloy.
Diaphragm Ø: 23 mm; bell Ø: 18 mm.
Tube treated rubber, PVC, crack resistant.
Tube impervious to outside noises, guaranteeing full transmission of sound, good auditive quality.
Tube diameter: outer Ø: 8-10mm, inner Ø: 4.2mm. Tube maximum length 60mm.
Sensitivity from 3.2dB to 26dB in a range from 50 to 1000Hz for cardiology.
Sensitivity 8.1dB in a range from 600 Hz to 1,500Hz for pneumology.
Arms: stainless steel with flexible spring.
Removable plastic earpieces.
Latex-free.
Designed for frequent and easy disassembly and disinfection with hospital-grade products.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x spare diaphragm.
1 x set spare earpieces.
ESTIMATED LIFE SPAN
Five years.
WARRANTY
Two years.
ENVIRONMENTAL CONDITIONS
Storage conditions: 0 - 50°C / 85% RH.
Operating conditions: 10 - 40°C / 85% RH.
WEIGHT AND VOLUME (Packaged)
Weight: 130 gr.
Volume: 69.53 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
There are no installation or commissioning requirements.
TRAINING REQUIREMENTS
Where staff has experience with these types of devices, no user training will be required.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements.
RELATED PRODUCTS
S0686000, Stethoscope,binaural,complete
S0686500, Stethoscope,foetal,Pinard
COMPONENT OF A KIT
S9902480 - NBK, Hospital, Module 3, Equipment
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
Self-declared CE under the EU MDR 2017/745 as Class I device.
SAFETY AND PRODUCT STANDARDS
- EN ISO 15223-1 (EN 980) Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements.
NOMENCLATURE
GMDN Code: 13755
Related Products