S0845030
Oxygen Analyzer, electrochem , handheld
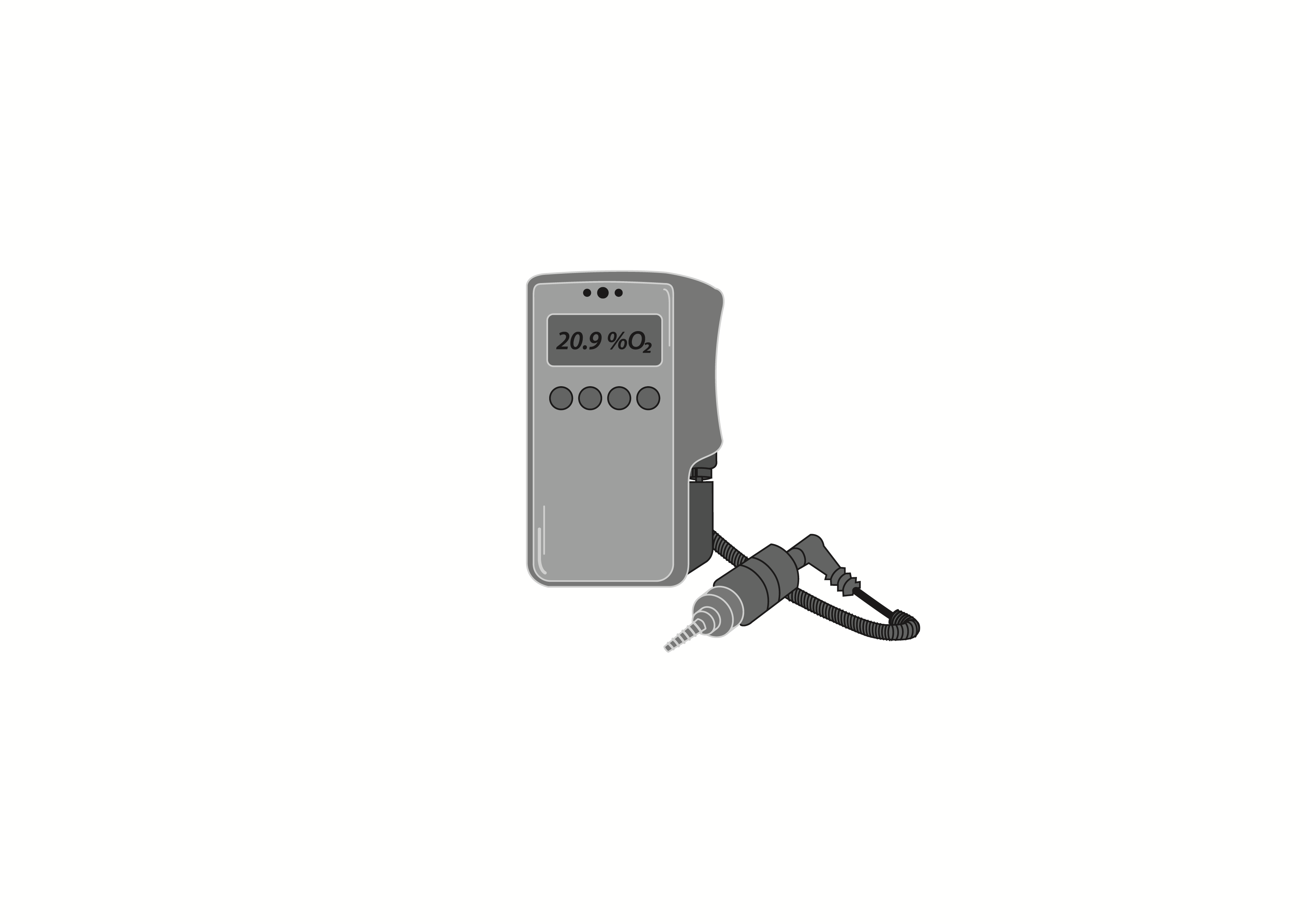
Handheld, battery powered device that measures the oxygen concentration in a flow of gas from a medical gas source or, with adapters, through a medical gas-flow device such as a ventilator or anaesthesia system, or within an environment such as oxygen hood and infant incubator.
Indicative Price 667.00 USD
GENERAL DESCRIPTION
Handheld, battery powered device that measures the oxygen concentrationin a flow of gas from a medical gas source or, with adapters, through a medical gas-flow device such as a ventilator or anaesthesia system, or within an environment such as oxygen hood and infant incubator.
TECHNICAL SPECIFICATIONS
Operational characteristics:
Handheld oxygen analyzer for continuous measurement of the oxygen concentration from a medical gas source and in an environment.
Electrochemical cell oxygen sensing technology.
O2 measurement includes the range 0.0 - 100%.
O2 resolution: 0.1 %.
O2 accuracy: within ± 3 %.
Suitable for measuring gas supply with pressure up to 50 psi (344 kPa).
Response time: ≤ 20 s.
Warm-up time: none required
Replaceable oxygen sensor cell, expected sensor life >1,500,000 O2% hours over 2 years in typical applications.
Calibration and self-test mode, one point calibration 20.9% or 100%.
Internal calibration timer (weekly); alerts the operator, using a calibration icon on the LCD display, to perform a unit calibration one week after previous calibration.
Low and high oxygen concentration alarm system, with flashing LED and audible indication of alarm conditions.
Display visualizing O2 concentration, system messages and battery status.
Electrical characteristics:
Operated by battery power supply.
Internal replaceable batteries, four AA alkaline batteries, single use.
Battery life: approximately 5,000 hours with typical use.
Sleep mode operation to extend battery life; in sleep mode operation, the unit will time-out after 90 seconds to a battery saving condition. In sleep mode, the device will continue to monitor the oxygen level and will activate the alarm if any alarm condition occurs.
Casing and cleaning:
IPX2 ingress protection rating.
Suitable for cleaning and disinfection with hospital-grade cleaning products.
SUPPLIED WITH
1 x T-Adapter.
1 x set single use batteries (4 AA batteries).
Instructions for Use supplied in English. (Soft copies in French, German, Dutch, Italian and Spanish, publicly available on the Manufacturer Website).
Certificate of Test and Certificate of Conformity available upon request, at the time of ordering.
List of equipment and procedures required for local calibration and routine maintenance provided in the Instructions for Use.
List of common spares and accessories, with part numbers, available in the Instructions for Use.
Contact details of manufacturer available in the Instruction for Use.
ENVIRONMENTAL CONDITIONS
Capable of being stored continuously in ambient temperature from -15 °C to 50 °C, RH from 0% to 95% and elevation from 2 to 1948 m.
Capable of operating continuously in ambient temperature from 15 °C to 40 °C, RH from 0% to 95%, and elevation from 2 to 1948 m.
ESTIMATED LEAD TIME
6 weeks
WARRANTY
Includes two-year standard warranty, dated from the completion of the terms of delivery.
WEIGHT & VOLUME
Weight: 0.77 kg (packaged)
Volume: 0.004 m³ (packaged)
MATERIAL SAFETY DATA SHEET INFORMATION (MSDS)
Proper Shipping Name: Corrosive liquid, toxic, n.o.s. (Acetic acid solution, Lead acetate)
Hazard Class: 8(6.1)
UN Number: UN2922
Packaging Group: III
REGULATION & CONFORMITY REQUIREMENTS
CE mark conforming to Medical Device Directive 93/42/EEC
CE Certificate (class IIb)
SAFETY & PRODUCT STANDARDS
Complies with the following standards:
EN ISO 13485:2016 Medical devices -Quality management systems -- Requirements for regulatory purposes
EN ISO 14971:2012 Medical devices — Application of risk management to medical devices
EN 60601-1:2006/A1:2013 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
EN 60601-1-2:2015 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral Standard: Electromagnetic disturbances - Requirements and tests
IEC 60601-1-8:2006/AMD1:2012 Medical electrical equipment - Part 1-8: General requirements for basic safety and essential performance - Collateral Standard: General requirements, tests and guidance for alarm systems in medical electrical equipment and medical electrical systems
EN 1041:2008 Information supplied by the manufacturer of medical devices
EN ISO 15223-1:2016 Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements
EN ISO 10993-1:2009 Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process
ISO 80601-2-55:2011 Medical electrical equipment — Part 2-55: Particular requirements for the basic safety and essential performance of respiratory gas monitors.
EN 62304:2006 Medical device software - Software life-cycle processes
ISTA 2A (2011) Packaged-Products weighing 150 lb (68 kg) or Less
Related Products